Applied Physics
Vol.
10
No.
01
(
2020
), Article ID:
33942
,
9
pages
10.12677/APP.2020.101009
Preparation and Luminescence Properties of Pr3+ doped YBO3 and Y3BO6 Phosphors
Wenhai Gao, Hong Zhou, Boqi Zhang, Shuang Li*
College of Science, Changchun University of Science and Technology, Changchun Jilin
Received: Dec. 25th, 2019; accepted: Jan. 7th, 2020; published: Jan. 14th, 2020

ABSTRACT
A series of Pr3+ doped YBO3 and Y3BO6 phosphors were prepared via solid-state reaction. XRD, SEM, PLE and PL spectra were used to characterize samples. The results revealed that the dosage of B plays an important role in the formation of the final product and the samples were irregular and granular. Under UV excitation, Y3BO6:Pr3+ phosphors showed strong red luminescence (1D2→3H4), while the blue-green emission from 3P0 was quenched. The strong luminescence region of YBO3:Pr3+ is distributed in the ultraviolet region (250 - 330 nm). In the visible region, the luminescence from the 3P0 level is weak. The analysis of luminescence mechanism shows that because Y3BO6 has higher phonon energy than YBO3, Y3BO6:Pr3+ is dominated by 1D2 luminescence in the visible region, and the luminescence from the 3P0 level is quenched, while the luminescence from the 3P0 to ground state can be observed in YBO3.
Keywords:YBO3, Y3BO6, Pr3+, Luminescence

Pr3+掺杂YBO3和Y3BO6荧光粉的 制备及光学性能研究
高文海,周红,张渤琦,李霜*
长春理工大学理学院,吉林 长春
收稿日期:2019年12月25日;录用日期:2020年1月7日;发布日期:2020年1月14日

摘 要
采用高温固相法合成了系列YBO3:Pr3+和Y3BO6:Pr3+荧光粉。用XRD、SEM、激发和发射光谱对样品进行了表征分析。结果表明:硼源的用量决定了最终产物的相结构,样品呈现不规则的颗粒状。在紫外激发下,Y3BO6:Pr3+荧光粉展现了强烈的红色发光(1D2→3H4),而与3P0相关的蓝绿光发射发生猝灭。YBO3:Pr3+荧光粉的强发光区分布在紫外区(250~330 nm),在可见光区发光较弱,主要发光来自3P0能级。发光机理分析表明由于Y3BO6比YBO3具有更高的声子能量,因此在可见光区,Y3BO6以1D2发光为主,3P0部分发生淬灭,而在YBO3中可观察到3P0→基态跃迁发光。
关键词 :YBO3,Y3BO6,Pr3+,发光

Copyright © 2020 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
稀土离子掺杂的硼酸盐发光材料因具有优异的光学特性、优良的化学性能和热稳定性,使其在显示和照明领域有广泛和重要的应用 [1] - [5]。硼酸盐结构类型丰富,在Ln2O3-B2O3二元体系中,包括含氧硼酸盐Ln3BO6,正硼酸盐LnBO3,偏硼酸盐Ln(BO2)3 [6]。YBO3作为优异的发光基质,具有稳定性高、毒性小、发光强度高及高光损伤阈值等一系列优良性能。然而,稀土离子掺杂YBO3材料,因O2+→Ln3+电荷跃迁带位于深紫外区域,其通常作为紫外发光材料使用,Pr3+掺杂YBO3材料在可见区的发光性能研究较少。
1997年Y3BO6晶体结构首次被报道,相比于YBO3,Y3BO6结构中Y元素同时存在YO7和YO8两种构型,B元素同时存在BO3和B2O5两种硼酸盐基团 [7]。YBO3和Y3BO6一般以H3BO3作为硼源,采用高温固相法进行制备。但由于H3BO3在高温下具有挥发性,YBO3和Y3BO6的制备很难得到纯相,而关于H3BO3用量对最终产物的影响鲜有报道。
Pr3+具有丰富的能级结构和优异的发光性能,使其得到了广泛应用。其中4f5d-4f2主要应用于紫外发光材料 [8] [9],1D2-3H4和3P0-3F2红色发光主要应用于荧光粉领域 [10] [11],3P1和3P0热耦合能级应用于温度传感领域 [12] [13]。此外,Pr3+发光峰的位置和发光效率受基质材料影响很大。不同的基质材料会对3P0相关的发光产生不同程度的淬灭现象,进而导致发光特性的显著不同。由于YBO3和Y3BO6具有不同的声子能量,这将使Pr3+在两个样品中发光产生显著的差异。在本论文中,我们以正硼酸盐YBO3和含氧硼酸盐Y3BO6作为基质,分析Pr3+在两种基质中的能级跃迁过程和发光特点。
2. 实验
采用高温固相法制备YBO3:Pr3+和Y3BO6:Pr3+系列荧光粉。由于硼源在高温下易挥发导致B不足,我们首先研究H3BO3用量对最终产物的影响,Y和B的化学计量比分别为3/1、3/1.5、3/2、1/1、1/1.5、1/2。将称量好的原料放入玛瑙研钵中充分研磨,然后将研磨好的样品,放入管式炉中进行第一步煅烧,煅烧温度为850℃,保温时间6 h。煅烧结束后,取出样品再次研磨。再进行第二步煅烧,在CO氛围下,煅烧温度1200℃,保温时间8 h,随炉降温得到最终样品。
样品的XRD数据采用Rigaku·D/max2500型(日本理学)粉末衍射仪进行收集。仪器参数:Cu靶,Kα1辐(λ = 0.15406 nm),扫描范围为10˚~90˚。荧光粉的表面形貌由JSM-6010LA型扫描电子显微镜(日本电子)进行表征。样品在室温下的激发光谱和发射光谱采用Shimadzu RF-5301pc型荧光光谱仪进行测量。色坐标的绘制采用CIE1931色坐标软件对样品的光谱数据进行计算。
3. 结果与讨论
3.1. XRD物相分析
图1是采用高温固相法制备的不同Y与B摩尔比获得产物的XRD结果。从图1可以看出,随着硼源比例的增加,将会得到不同的最终产物,这是由于高温下H3BO3的挥发使硼源减少所致。当Y/B的值为3/1时,产物主相是Y2O3。当Y/B的值为3/1.5时,得到的产物为Y3BO6单相。当Y/B的值比例处于3/2~1/1.5之间时,得到产物为YBO3和Y3BO6混相。当Y/B的值为1/2时,得到的产物为YBO3单相。
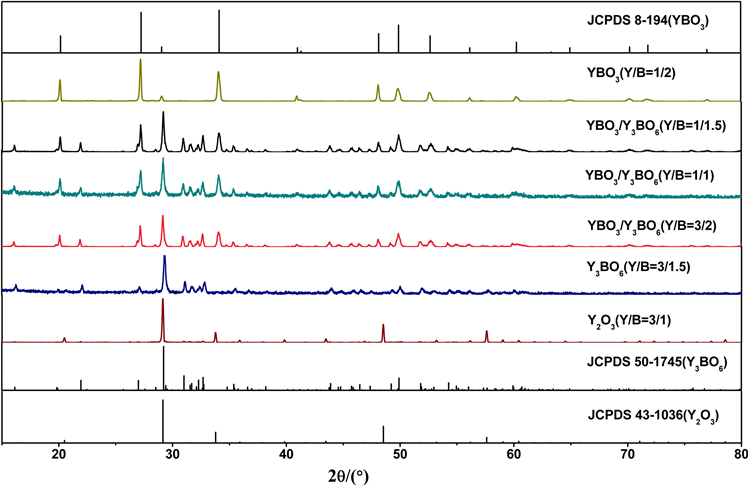
Figure 1. XRD patterns of the products with different Y/B ratios
图1. 不同Y/B比值的产物的XRD图谱
综上,我们分别以Y/B = 2/1和Y/B = 1/2时准备了系列Y3BO6和Y3BO6样品,并进行了不同浓度的Pr3+掺杂。图2是YBO3:xPr3+ (x = 0.001, x = 0.005, x = 0.01, x = 0.015, x = 0.02)系列样品的XRD图谱。结果显示不同掺杂浓度样品的所有衍射峰与YBO3标准的PDF卡片(JCPDS 8-194)很好的匹配,没有杂峰出现。说明产物具有基质YBO3的晶体结构,Pr3+离子进入YBO3主晶格后,没有对YBO3基质的晶格结构产生影响。
图3是制备的不同掺杂浓度的Y3BO6:xPr3+ (x = 0.00, x = 0.005, x = 0.01, x = 0.015, x = 0.02)荧光粉的XRD图谱。图中所有样品的衍射峰与Y3BO6标准的PDF卡片(JCPDS 50-1745)相匹配,无其它杂峰,说明Pr3+掺入Y3BO6晶格中,不影响其晶体结构。
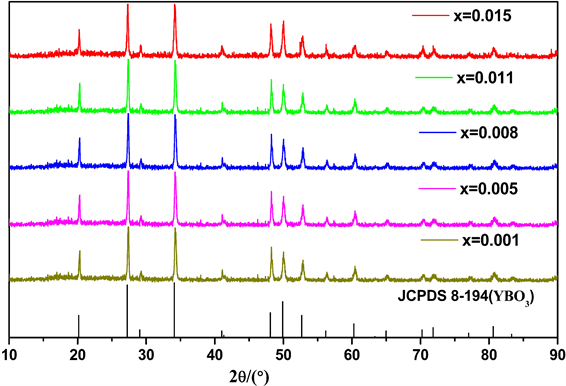
Figure 2. XRD patterns of the prepared products for YBO3:xPr3+ (x = 0.001, x = 0.005, x = 0.08, x = 0.011, x = 0.015)
图2. YBO3:xPr3+ (x = 0.001, x = 0.005, x = 0.08, x = 0.011, x = 0.015)样品的XRD图谱

Figure 3. XRD patterns of the prepared products for Y3BO6:xPr3+ (x = 0.00, x = 0.005, x = 0.01, x = 0.015, x = 0.02)
图3. Y3BO6:xPr3+ (x = 0.00, x = 0.005, x = 0.01, x = 0.015, x = 0.02)样品的XRD图谱
3.2. SEM结果分析
图4是YBO3:1% Pr3+、Y3BO6:1.5% Pr3+和YBO3/Y3BO6:0.8% Pr3+样品的扫描电镜图(SEM)。从图中可以看出,YBO3:1% Pr3+颗粒的形状接近圆形,Y3BO6:1.5% Pr3+颗粒呈现不规则的颗粒状,YBO3/Y3BO6:0.8% Pr3+颗粒表面比较粗超,且三个样品都出现了团聚现象,平均粒径约为1 μm。
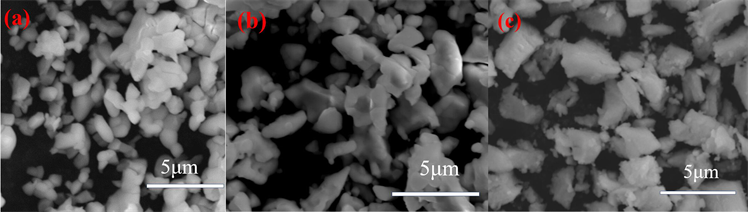
Figure 4. SEM images of (a) YBO3:1% Pr3+, (b) Y3BO6:1.5% Pr3+ and (c) YBO3/Y3BO6:0.8% Pr3+ phosphors
图4. (a) YBO3:1% Pr3+、(b) Y3BO6:1.5% Pr3+和(c) YBO3/Y3BO6:0.8% Pr3+样品的扫描电镜图
3.3. 光谱特性分析
图5(a)是YBO3:xPr3+ (x = 0.001, x = 0.005, x = 0.01, x = 0.015, x = 0.02)在521 nm监测下的激发光谱,230~260 nm的宽带光谱为O-B键的电荷迁移带。图5(b)是在250 nm激发下的发射光谱。在500~650 nm的范围内,有3个发光峰,分别归属于3P1→3H5 (521 nm)、3P0→3H5 (548 nm)和1D2→3H4 (597 nm)电子跃迁。在290~330 nm的发射带,归属于Pr3+的4f15d1→3F2电子跃迁。随着Pr3+掺杂量的增加,YBO3:Pr3+的4个发光峰的发射强度都逐渐增加。当Pr3+的掺杂量增值x = 0.01时,Pr3+的发光强度增至最大,之后由于浓度淬灭效应造成发光强度下降。
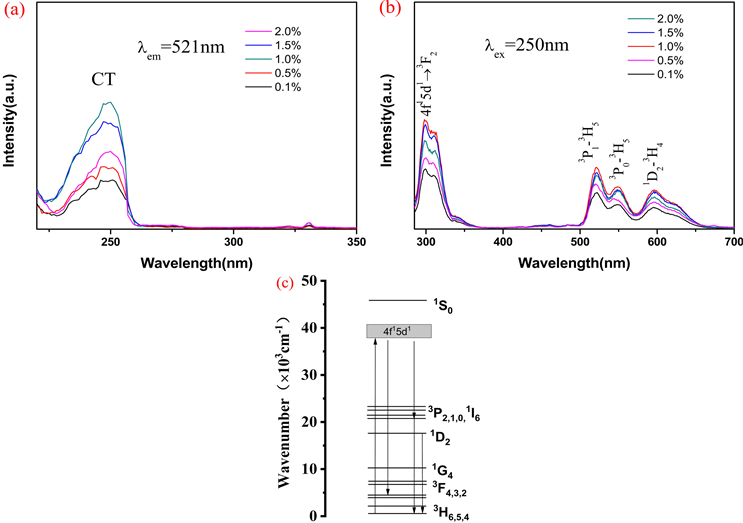
Figure 5. PLE (a) and PL (b) spectra of YBO3:xPr3+ and energy level diagram of Pr3+ (c)
图5. YBO3:xPr3+的(a)激发光谱、(b)发射光谱和(c) Pr3+的能级示意图
图6为Y3BO6:xPr3+ (x = 0.00, x = 0.005, x = 0.01, x = 0.015, x = 0.02)系列荧光粉的发射和激发光谱和Pr3+的能级示意图。根据图6(a)所示激发光谱可以看出,在监测波长630 nm下,位于232 nm的吸收峰归属于4f2→4f15d1跃迁,在250~300 nm存在一个很宽的电荷跃迁吸收带,归属于O→B的电荷跃迁。根据上述分析测量发射光谱时选择的激发波长为λex = 292 nm,发射光谱如图6(b)所示,Y3BO6:xPr3+荧光粉的发光峰主要集中在575~675 nm范围,随着Pr3+掺杂量的增加,630 nm处的Pr3+的发射强度逐渐增加。当Pr3+的掺杂量增至x = 0.015时,Pr3+的发光强度增至最大,之后由于浓度淬灭效应造成发光强度下降。分别位于605 nm、630 nm和645 nm处的三个主要发射峰均是Pr3+离子4f轨道电子的特征发射峰,归属于3P0→3H6 (602 nm)、1D2→3H4 (630 nm)和1D2→3H5 (653 nm)电子跃迁,其中位于630 nm的发射峰最强。
我们测量了YBO3/Y3BO6:Pr3+混合基质荧光粉的激发和发射光谱。激发光谱选择监测波长为630 nm,从图7(a)看出,在240 nm到380 nm的监测区间中,有2个宽带激发,位于303 nm的吸收峰归属于4f2→4f15d1跃迁,在334 nm的吸收峰归属于O→B的电荷跃迁。这与Y3BO6:Pr3+的激发光谱很接近,不同于YBO3:Pr3+的激发光谱。图7(b)是在300 nm激发波长下,测得YBO3/Y3BO6:Pr3+样品的发射光谱。整个光谱覆盖365 nm紫外区、500 nm绿光区和590~660 nm红光区组成。其中紫外发射和红光发射较强,500 nm绿光发射较弱。其中位于602 nm、630 nm和653 nm的发光峰分别对应于1D2→3H4 (602 nm)、3P0→3H6 (630 nm)和1D2→3H5 (653 nm)能级跃迁。随着Pr3+掺杂量的增加,Pr3+的发射强度逐渐增加。当Pr3+的掺杂量增值x = 0.015时,Pr3+的发光强度增至最大,之后由于浓度淬灭效应造成发光强度下降。通过与单基质YBO3:Pr3+和Y3BO6:Pr3+的发射光谱的对比,可以发现在混合基质YBO3/Y3BO6:Pr3+中红光发射主要来自于Y3BO6:Pr3+的贡献,而紫外区和绿光发射与YBO3:Pr3+有关,所以可以通过调整YBO3和Y3BO6比例实现多色发光。

Figure 6. PLE (a) and PL (b) spectra of Y3BO6:xPr3+ and energy level diagram of Pr3+ (c)
图6. Y3BO6:xPr3+的(a)激发光谱、(b)发射光谱和(c) Pr3+的能级示意图

Figure 7. PLE (a) and PL (b) spectra of YBO3/Y3BO6:xPr3+
图7. YBO3/Y3BO6:xPr3+的(a)激发光谱和(b)发射光谱
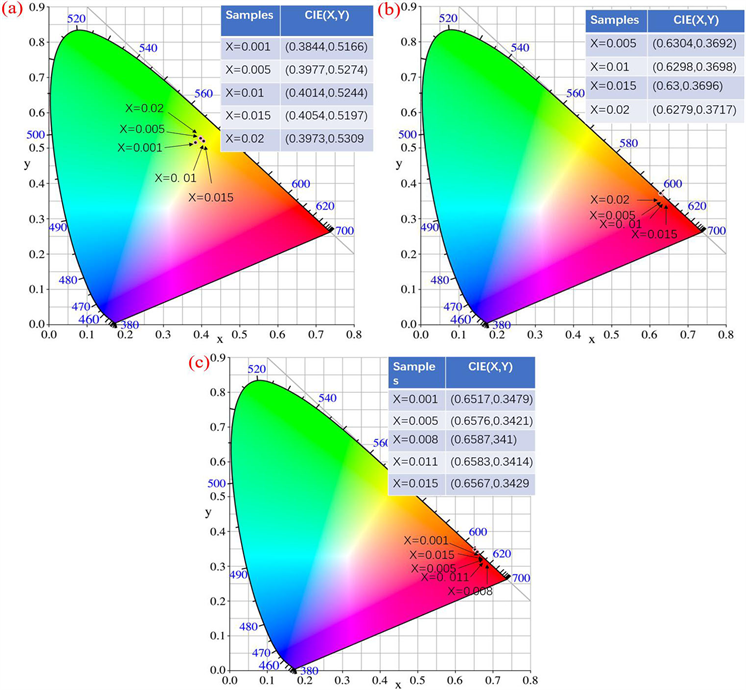
Figure 8. CIE chromaticity coordinates of YBO3:xPr3+ (a), Y3BO6:xPr3+ (b) and YBO3/Y3BO6:xPr3+ (c) phosphors
图8. YBO3:xPr3+ (a)、Y3BO6:xPr3+ (b)和YBO3/Y3BO6:xPr3+ (c)系列荧光粉的色度坐标
3.4. CIE色度图分析
YBO3:xPr3+、Y3BO6:xPr3+、YBO3/Y3BO6:xPr3+和荧光粉在各自最佳激发条件下的CIE坐标如图8所示。结果表明,Y3BO6:xPr3+和YBO3/Y3BO6:xPr3+荧光粉的CIE坐标都在红色区域,荧光粉的颜色坐标与理想红色荧光粉的颜色坐标(0.65、0.35)非常接近,可以作为白色照明和显示领域的红色荧光粉,而YBO3:xPr3+荧光的CIE坐标主要位于黄绿色区域。
3.5. 发光机理分析
对比YBO3:Pr3+和Y3BO6:Pr3+两个单基质的发光特点可知,二者主要区别是YBO3:Pr3+有紫外发光,而Y3BO6:Pr3+强发光集中在可见光区。另外对比二者在可见光区的发光区别,YBO3:Pr3+发光以3P0能级为主,而Y3BO6:Pr3+发光以1D2能级为主,3P0能级发生淬灭。这是由于在Y3BO6:Pr3+中,到达3P0能级的电子通过多声子弛豫跃迁过程无辐射衰减到1D2能级。多声子弛豫的几率可由Dijk和Schuurmans的能隙公式得到 [14]:
(1)
上式中βel和α是与基质相关的常数, 是2个激发态间能量差, 是基质中的最高能量的声子振动频率。对于硼酸盐体系, 和 [15]。3P0→1D2间的能量差约为3500 cm−1 [16],通过红外光谱可以得到Y3BO6的最高能量的声子振动频率约为1400 cm−1 [17],由(1)式可以得到Y3BO6:Pr3+中3P0到1D2的多声子弛豫的几率为 ,大于3P0能级向基态辐射跃迁的几率( )。所以在Y3BO6:Pr3+和YBO3/Y3BO6:Pr3+中,更容易发生3P0→1D2非辐射跃迁,再产生以1D2为主的红光发射。而YBO3的声子能量约为1000 cm−1 [17],要比Y3BO6小很多,通过计算可以得到YBO3:Pr3+中3P0到1D2的多声子弛豫的几率为 ,与3P0能级向基态辐射跃迁的几率为同一数量级,因此可观察到以3P0发射为主的发光。
4. 结论
本文采用高温固相法合成了系列Pr3+掺杂YBO3和Y3BO6荧光粉,通过XRD、SEM、发射和激发光谱对样品进行了表征分析。在YBO3和Y3BO6的制备过程中,通过调整Y/B的值,我们找到了YBO3和Y3BO6的最佳制备方案。在近紫外激发下,Y3BO6:Pr3+和YBO3/Y3BO6:Pr3+荧光粉的颜色坐标接近理想的红色发光,而YBO3:Pr3+荧光粉的颜色坐标主要位于黄绿色区域,表明产物在近紫外激发的白光LED领域具有潜在的应用价值。发光机理分析表明由于Y3BO6比YBO3具有更高的声子能量,导致3P0→1D2的非辐射跃迁几率大于3P0→基态跃迁几率,因此在可见光区,Y3BO6以1D2发光为主,3P0部分发生淬灭,而在YBO3中可观察到3P0→基态跃迁发光。
文章引用
高文海,周 红,张渤琦,李 霜. Pr3+掺杂YBO3和Y3BO6荧光粉的制备及光学性能研究
Preparation and Luminescence Properties of Pr3+ doped YBO3 and Y3BO6 Phosphors[J]. 应用物理, 2020, 10(01): 76-84. https://doi.org/10.12677/APP.2020.101009
参考文献
- 1. Wang, C. and Yan, B. (2008) Sol-Gel Synthesis and Photoluminescence of RE3BO6: Eu3+/Tb3+ (RE = Y, Gd) Microcrystalline Phosphors from Hybrid Precursors. Journal of Non-Crystalline Solids, 354, 962-969. https://doi.org/10.1016/j.jnoncrysol.2007.08.029
- 2. Zhang, X.W., Zhao, Z., Zhang, X., Maratheet, A., Cordes, D.B., Weeksal, B. and Chaudhuri, J. (2013) Tunable Photoluminescence and Energy Transfer of YBO3: Tb3+, Eu3+ for White Light Emitting Diodes. Journal of Materials Chemistry C, 1, 7202-7207. https://doi.org/10.1039/c3tc31200c
- 3. Zhang, Z.W., Wang, L.J., Chu, X.J., Zhang, P., Cao, Y.J., Xi, Y.R., Chen, W.G. and Wang, D.J. (2016) High-Brightness Ca9NaGd0.667(1-x)(PO4)7:xEu3+ Red Phosphor for NUV Light-Emitting Diodes Application. Journal of Alloys and Compounds, 695, 3220-3224. https://doi.org/10.1016/j.jallcom.2016.11.297
- 4. Kaur, S., Jayasimhadri, M. and Rao, A.S. (2017) A Novel Red Emitting Eu3+ Doped Calcium Aluminozincate Phosphor for Applications in w-LEDs. Journal of Alloys and Compounds, 697, 367-373.https://doi.org/10.1016/j.jallcom.2016.12.150
- 5. Peng, Y., Li, R.X., Cheng, H., Chen, Z., Li, H. and Chen, M.X. (2017) Facile Preparation of Patterned Phosphor-in-Glass with Excellent Luminous Properties through Screen-Printing for High-Power White Light-Emitting Diodes. Journal of Alloys and Compounds, 693, 279-284. https://doi.org/10.1016/j.jallcom.2016.09.197
- 6. Cohen-Adad, M.T., Aloui-Lebbou, O., Goutaudier, C., Panczer, G., Dujardin, C., Pedrini, C., Florian, P., Massiot, D., Gerard, F. and Kappenstein, C. (2000) Gadolinium and Yttrium Borates: Thermal Behavior and Structural Considerations. Journal of Solid State Chemistry, 154, 204-213. https://doi.org/10.1006/jssc.2000.8837
- 7. Lin, J.H., Zhou, S., Yang, L.Q., Yao, Q. and Su, M.Z. (1997) Structure and Luminescent Properties of Y17.33 (BO3)4(B2O5)2O16. Journal of Solid State Chemistry, 134, 158-163. https://doi.org/10.1006/jssc.1997.7567
- 8. Broxtermann, M., Engelsen, D.D., Fern, G.R., Harris, P., Ireland, T.G., Justel, T. and Silver, J. (2017) Cathodoluminescence and Photoluminescence of YPO4:Pr3+, Y2SiO5:Pr3+, YBO3:Pr3+, and YPO4:Bi3+. ECS Journal of Solid State Science and Technology, 6, 47-52. https://doi.org/10.1149/2.0051704jss
- 9. Drozdowski, W., Wojtowicz, A.J., Wisniewski, D., Szupryczynski, P., Janus, S., Lefaucheur, J.-L. and Gou, Z. (2004) VUV Spectroscopy and Low Temperature Thermoluminescence of LSO:Ce and YSO:Ce. Journal of Alloys and Compounds, 380, 146-150. https://doi.org/10.1016/j.jallcom.2004.03.016
- 10. Ma, S.Z., Feng, W.L., Chen, R. and Peng, Z.Q. (2017) KSr4(BO3)3:Pr3+: A New Red-Emitting Phosphor for Blue-Pumped White Light-Emitting Diodes. Journal of Alloys and Compounds, 700, 49-53. https://doi.org/10.1016/j.jallcom.2017.01.069
- 11. Tan, S.Y., Yang, P.P., Li, C.X., Wang, W.X., Wang, J., Zhang, M.L., J, X.Y. and Lin, J. (2010) Preparation, Characterization and Luminescent Properties of Spherical CaTiO3:Pr3+ Phosphors by Spray Pyrolysis. Solid State Sciences, 12, 624-629. https://doi.org/10.1016/j.solidstatesciences.2010.01.015
- 12. Lei, R.S., Luo, X.Y., Yuan, Z.Y., Wang, H.P., Huang, F.F., Deng, D.G. and Xu, S.Q. (2019) Ultrahigh-Sensitive Optical Temperature Sensing in Pr3+:Y2Ti2O7 Based on Diverse Thermal Response from Trap Emission and Pr3+ Red Luminescence. Journal of Luminescence, 205, 440-445. https://doi.org/10.1016/j.jlumin.2018.09.029
- 13. Zhou, S.S., Jiang, G.C., Wei, X.T., Duan, C.K., Chen, Y.H. and Yin, M. (2014) Pr3+-Doped β-NaYF4 for Temperature Sensing with Fluorescence Intensity Ratio Technique. Journal of Nanoscience and Nanotechnology, 14, 3739-3742. https://doi.org/10.1166/jnn.2014.8010
- 14. Van Dijk, J.M.F. and Schuurmans, M.F.H. (1983) On the Nonradiative and Radiative Decay Rates and a Modified Exponential Energy Gap Law for 4f-4f Transitions in Rare-Earth Ions. The Journal of Chemical Physics, 78, 5317-5323. https://doi.org/10.1063/1.445485
- 15. Van Dijk, J.M.F. and Schuurmans, M.F.H. (1984) On Radiative and Non-Radiative Decay Times in the Weak Coupling Limit. Physica B + C, 123, 131-155. https://doi.org/10.1016/0378-4363(84)90117-7
- 16. Chanthima, N., Boonin, K., Limsuwan, P. and Kaewkhao, J. (2013) Luminescence of Pr3+ in Bismuth Borate Glasses. Advanced Materials Research, 770, 59-63. https://doi.org/10.4028/www.scientific.net/AMR.770.59
- 17. Nair, R.G., Nigam, S., Sudarsan, V., Vatsa, R.K. and Jain, V.K. (2018) YBO3 versus Y3BO6 Host on Tb3+ Luminescence. Journal of Luminescence, 195, 271-277. https://doi.org/10.1016/j.jlumin.2017.11.038