Advances in Analytical Chemistry
Vol.
12
No.
03
(
2022
), Article ID:
55051
,
8
pages
10.12677/AAC.2022.123029
新型10-羟基喜树碱前药分子的制备与 性能研究
蔡丽娟,朱金丽,汤艳峰,孙广平*
南通大学化学化工学院,江苏 南通
收稿日期:2022年8月2日;录用日期:2022年8月12日;发布日期:2022年8月23日

摘要
本文以10-羟基喜树碱(HCPT, 10-Hydroxycamptothecin)作为前药分子,通过2,4-二硝基苯磺酰氯(DNS)的取代反应,成功构筑了一种具有荧光探针性能的喜树碱前药分子(DNS-HCPT)。荧光试验表明DNS-HCPT在谷胱甘肽(GSH)的作用下,能将前药分子HCPT快速地释放出来,发射出很强的黄色荧光,可用于肿瘤组织的追踪与识别。此外,HCPT作为喜树碱衍生物中抗癌效果最好之一,DNS-HCPT释放的HCPT还能被用于癌细胞的治疗,在癌细胞的诊断与治疗中具有潜在的应用价值。
关键词
10-羟基喜树碱前药分子,GSH刺激响应,荧光

The Preparation and Investigation of a Novel 10-Hydroxycamptothecin Prodrug
Lijuan Cai, Jinli Zhu, Yanfeng Tang, Guangping Sun*
College of Chemistry and Chemical Engineering, Nantong University, Nantong Jiangsu
Received: Aug. 2nd, 2022; accepted: Aug. 12th, 2022; published: Aug. 23rd, 2022

ABSTRACT
Prodrug DNS-HCPT was successfully prepared via the substitution reaction of 10-hydroxycamptothecin (HCPT) and 2,4-dinitrobenzenesulfonyl chloride (DNS), which could be used as a fluorescent probe. HCPT could be effectively released under glutathione (GSH) condition and realized strong yellow fluorescence emission, suggesting potential in tracking tumor tissues. Moreover, as one of the most effective anticancer camptothecin derivatives, the released HCPT could be utilized for cancer therapy, which was potential in cancer diagnosis and therapy.
Keywords:10-Hydroxycamptothecin Prodrug, GSH Stimulus Response, Fluorescence

Copyright © 2022 by author(s) and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/


1. 引言
癌症作为人类健康的重大威胁之一,对人们的身心健康产生了重大影响。为了克服癌症带来的危害,人们采用各种方法对其进行研究探索 [1] [2] [3]。前药策略,因其能够显著提高化疗药物的药代能力、药学和药效特性,在癌症治疗中获得了更多的关注 [4] - [9]。尤其是将具有刺激响应性能的控制单元修饰到前药上,不仅能显著提高药物的载药效率,还能获得具有肿瘤微环境响应性能的前药系统,智能释放抗癌药物,实现癌细胞治疗效果 [10] [11] [12] [13] [14]。与正常的组织细胞不同,肿瘤组织具有独特的物理化学特性,比如肿瘤细胞内具有较高浓度的过氧化氢,这主要是由于细胞内氧化还原动态失衡,从而导致细胞出现氧化应激态,过度产生活性氧(ROS),其浓度远高于正常细胞(约100倍),高浓度的ROS会导致细胞的永久死亡 [7] [15]。值得注意的是,为了维持细胞内氧化还原的稳定,即使是癌细胞也会产生更多的谷胱甘肽(GSH,内源性三肽)作为抗氧化剂来清除活性氧以避免氧化失衡 [7] [15]。由于高浓度的ROS,癌细胞内的GSH浓度也相应的高出正常细胞7~10倍 [15]。此外,研究表明,GSH不仅参与癌细胞的分裂增殖,还对细胞的多种生物过程起到至关重要的作用,比如:外源性物质的解毒过程与细胞的周期进程 [7]。因此,利用癌细胞内高浓度的GSH设计具有GSH响应性能的调控单元在癌症治疗中具有潜在的应用价值。
喜树碱作为一种天然抗癌药物,其衍生物已被广泛应用于各种癌细胞治疗。10-羟基喜树碱(HCPT, 10-Hydroxycamptothecin)抗癌机制与喜树碱相似,被认为是喜树碱衍生物中抗癌效果最好之一,其主要通过抑制拓扑异构酶来缓解DNA的扭转 [7] [16]。由于HCPT具有很强的荧光发射能力,因此,我们认为基于HCPT构筑具有GSH响应机制的前药分子不仅能通过HCPT的GSH刺激响应性荧光指示肿瘤组织的位置,实时追踪药物释放过程,还能作为前药分子,显著提高载药效率,实现癌细胞的有效治疗。
为此,我们通过取代反应设计合成了具有GSH响应性能的HCPT前药分子(DNS-HCPT)。在前药分子DNS-HCPT中,2,4-二硝基苯磺酰基(DNS)通过取代反应与HCPT共价相连,且由于DNS的吸电子效应致使HCPT自身荧光发生猝灭,但是在高浓度的GSH作用下,DNS会发生分子内重排并从DNS-HCPT上脱离,将HCPT释放出来,此时的HCPT不仅能高效治疗肿瘤组织,还能通过自身的荧光实现肿瘤组织的定位与诊断。GSH刺激响应释放结果表明,DNS-HCPT前药分子不仅能有效释放前药HCPT用于治疗癌细胞,其GSH响应后的荧光变化还可用于追踪药物释放过程及肿瘤位置,在肿瘤治疗与诊断中具有潜在价值(图1)。
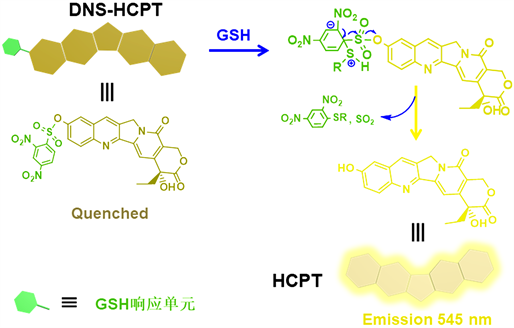
Figure 1. GSH responsive illustration of 10-hydroxycamptothecin prodrug (DNS-HCPT)
图1. 10-羟基喜树碱前药分子(DNS-HCPT)的GSH响应示意图
2. 实验部分
2.1. 试剂与仪器
10-羟基喜树碱(HCPT, 99%)、2,4-二硝基苯磺酰氯(DNS, 98%),安耐吉化学;三乙胺(TEA)、碳酸氢钠、无水硫酸钠、二氯甲烷(DCM)、石油醚(PE)、乙酸乙酯(EA),分析纯,南京化学试剂有限公司;薄层层析硅胶,300~400目,山东青岛海洋。
Bruker 400 MHz核磁共振仪,瑞士Bruker公司;Hitachi F-7000荧光光谱仪,日本日立公司;IKA RV 10旋转蒸发仪,德国IKA公司;WFH-203B暗箱台式三用紫外分析仪,上海精密仪器仪表有限公司。
2.2. 10-羟基喜树碱前药分子(DNS-HCPT)的合成
合成路线如图2所示:

Figure 2. Synthesis route of DNS-HCPT
图2. DNS-HCPT的合成路线
将10-羟基喜树碱(0.93 g, 2.55 mmol)溶解在100 mL的二氯甲烷中,加入0.8 g的三乙胺,冰浴降温20 min,接着将溶有2,4-二硝基苯磺酰氯(0.8 g, 3 mmol)的50 mL二氯甲烷恒压滴加至反应液中,滴加时间超过1.5 h,滴加结束,继续搅拌反应3 h。反应结束,反应液用饱和碳酸氢钠洗涤三次,收集有机相,用无水硫酸钠干燥过夜。过滤收集滤液,直接硅胶拌样,以石油醚/乙酸乙酯(v/v = 1:3)作为流动相,通过柱层析纯化得黄色固体产物DNS-HCPT (0.91 g, 1.53 mmol, 60%)。
2.3. 10-羟基喜树碱前药分子(DNS-HCPT)的核磁测试
在纯化得到目标产物DNS-HCPT后,以DMSO-d6作为溶剂,通过Bruker 400 MHz核磁共振仪对DNS-HCPT的氢谱、碳谱进行检测分析(图3和图4),数据和图如下:1H NMR (400 MHz, DMSO-d6, 298 K) δ (ppm): 9.16 (d, J = 2.4 Hz, 1H), 8.72 (s, 1H), 8.58 (dd, J = 8.4, 2.0 Hz, 1H), 8.31 (d, J = 8.8 Hz, 1H), 8.25 (d, J = 8.8 Hz, 1H), 8.04 (d, J = 2.8 Hz, 1H), 7.66 (dd, J = 9.2, 2.4 Hz, 1H), 7.35 (s, 1H), 5.43 (s, 2H), 5.30 (s, 2H), 1.86~1.81 (m, 2H), 0.87 (t, J = 7.2 Hz, 3H).13C NMR (100 MHz, DMSO-d6, 298 K) δ (ppm): 172.9, 157.2, 154.3, 152.1, 150.4, 148.7, 147.1, 147.0, 145.5, 134.2, 132.3, 132.3, 131.5, 131.0, 128.7, 128.0, 125.3, 121.7, 121.1, 120.1, 97.6, 72.8, 65.7, 50.7, 30.7, 8.2.

Figure 3. 1H NMR spectrum (400 MHz, DMSO-d6, 298 K) of DNS-HCPT
图3. DNS-HCPT的氢谱(400 MHz, DMSO-d6, 298 K)
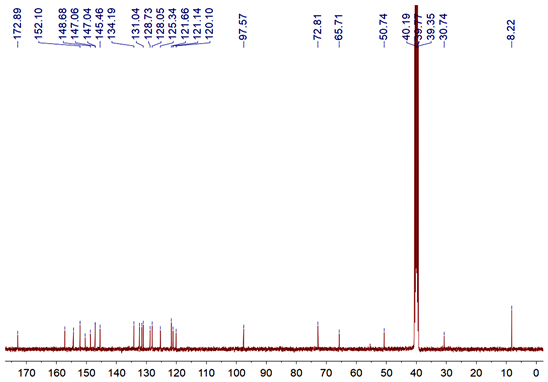
Figure 4. 13C NMR spectrum (100 MHz, DMSO-d6, 298 K) of DNS-HCPT
图4. DNS-HCPT的碳谱(100 MHz, DMSO-d6, 298 K)
3. 结果与讨论
3.1. DNS-HCPT的GSH刺激响应性研究
根据相关文献报道 [17] [18],前药分子DNS-HCPT中的2,4-二硝基苯磺酰基(DNS)部分对GSH具有显著的刺激响应性,可以使DNS-HCPT中的DNS重排离去,接下来首先对DNS-HCPT的GSH刺激响应性进行研究。
首先,配制0.2 mM的DNS-HCPT水溶液(含少量DMSO助溶),通过荧光光谱检测发现几乎没有荧光信号,此时在365 nm激发下也观察不到任何荧光,说明此时前药分子DNS-HCPT非常稳定,HCPT不能被释放出来(图5和图6)。然而,当向DNS-HCPT溶液中加入0.2 mM的GSH溶液后,1 min后可以检测到荧光强度为828 (545 nm处),说明HCPT被释放出来了,并且随着时间的增加,荧光强度越来越强,8 min后甚至可以达到2288 (545 nm处),说明前药分子DNS-HCPT具有明显的GSH刺激响应性(图5)。
更为重要的是,通过8 min的GSH刺激响应释放,我们可以观察到显著的黄色荧光,说明大部分前药HCPT被释放出来了,相较于GSH刺激响应释放前,荧光强度足足提高了48倍(图6)。上述荧光实验表明前药分子DNS-HCPT不仅具有显著的GSH刺激响应性能,其释放的前药分子HCPT还能够被用于追踪识别肿瘤组织的高浓度GSH环境,同时对癌细胞进行治疗,在肿瘤的诊治结合中具有潜在的价值。
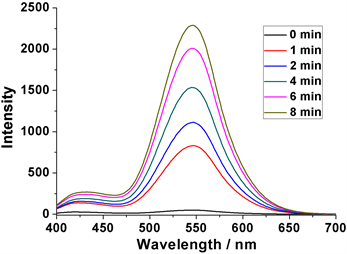
Figure 5. Fluorescence spectra of DNS-HCPT (0.2 mM) with GSH (0.2 mM) in different time
图5. DNS-HCPT (0.2 mM)在GSH (0.2 mM)作用下不同时间段的荧光图谱
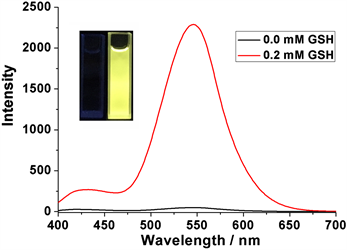
Figure 6. Fluorescence spectra of DNS-HCPT (0.2 mM) with 0.0 and 0.2 mM GSH
图6. DNS-HCPT (0.2 mM)在0.0和0.2 mM GSH作用下的荧光谱图
3.2. DNS-HCPT的释放研究
在确定前药分子DNS-HCPT的GSH刺激响应性能后,接着对DNS-HCPT (0.2 mM)的GSH刺激响应释放进行了深入研究。如图7所示,在没有GSH的情况下,前药分子DNS-HCPT能够一直保持稳定,几乎没有任何的HCPT荧光信号能够被检测到。然而,当加入0.1 mM的GSH后,可以发现HCPT的荧光信号显著增强,随着时间的增加逐渐达到平衡,说明DNS-HCPT在GSH的作用下成功地释放出了前药分子HCPT。值得注意的是,随着GSH浓度从0.1 mM增加到0.2 mM和0.5 mM,HCPT的释放量也显著地从48%增加到了80%和90%,说明该前药分子具有很好的GSH刺激响应性能。这些释放结果说明,DNS-HCPT不仅具有显著的GSH刺激响应性能,在高浓度GSH下还能高效地释放前药分子HCPT,在癌细胞的诊治中具有重要价值。
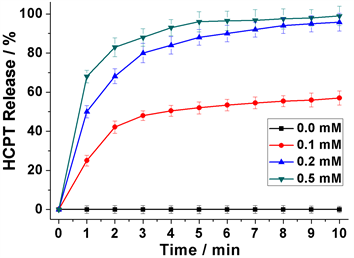
Figure 7. Time-dependent HCPT release at different GSH concentrations
图7. DNS-HCPT (0.2 mM)在不同GSH浓度下的释放曲线
3.3. DNS-HCPT的释放机理研究
谷胱甘肽(GSH)是一种谷氨酸、半胱氨酸和甘氨酸形成的含有巯基的三肽(R-SH)。文献表明 [5] [7] [8],当加入GSH后,R-SH中的巯基会进攻DNS,发生取代反应,同时DNS中的磺酰基会进行重排,最终以SO2的形式离去,而GSH通过巯基最终被修饰到磺酰基的位置,从而把前药HCPT给释放出来,实现GSH的刺激响应释放(图8)。

Figure 8. GSH release mechanism of DNS-HCPT
图8. DNS-HCPT的GSH释放机理
4. 结论
我们以10-羟基喜树碱(HCPT, 10-Hydroxycamptothecin)作为前药分子,通过取代反应在HCPT的10-羟基位置成功修饰了2,4-二硝基苯磺酰基(DNS),得到了具有诊治结合功能的喜树碱前药分子(DNS-HCPT)。由于DNS的分子内抑制作用,前药分子DNS-HCPT不发射荧光,但是在谷胱甘肽(GSH)的作用下,DNS-HCPT不仅能将前药分子HCPT快速地释放出来,其释放的HCPT还能发射出很强的黄色荧光,这样显著的荧光变化可被用于肿瘤组织的追踪与识别。更为重要的是,HCPT作为喜树碱衍生物中抗癌效果最好之一,DNS-HCPT释放的HCPT还能被用于癌细胞的治疗。因此,该前药分子DNS-HCPT在癌细胞的诊断与治疗中具有潜在的应用价值。
基金项目
本项目由国家自然科学基金(No. 22075152)支持。
文章引用
蔡丽娟,朱金丽,汤艳峰,孙广平. 新型10-羟基喜树碱前药分子的制备与性能研究
The Preparation and Investigation of a Novel 10-Hydroxycamptothecin Prodrug[J]. 分析化学进展, 2022, 12(03): 232-239. https://doi.org/10.12677/AAC.2022.123029
参考文献
- 1. Ahles, T.A. and Root, J.C. (2018) Cognitive Effects of Cancer and Cancer Treatments. Annual Review of Clinical Psychology, 14, 425-451.
https://doi.org/10.1146/annurev-clinpsy-050817-084903 - 2. Qin, S.Y., Zhang, A.Q., Cheng, S.X., et al. (2017) Drug Self-Delivery Systems for Cancer Therapy. Biomaterials, 112, 234-247.
https://doi.org/10.1016/j.biomaterials.2016.10.016 - 3. He, Q. and Shi, J. (2014) MSN Anti-Cancer Nanomedicines: Chemotherapy Enhancement, Overcoming of Drug Resistance, and Metastasis Inhibition. Advanced Materials, 26, 391-411.
https://doi.org/10.1002/adma.201303123 - 4. Hu, X.Y., Gao, L., Mosel, S., et al. (2018) From Supramolecular Vesicles to Micelles: Controllable Construction of Tumor-Targeting Nanocarriers-Based on Host-Guest Interaction between a Pillararene-Based Prodrug and a RGD- Sulfonate Guest. Small, 14, 1803952-1803962.
https://doi.org/10.1002/smll.201803952 - 5. Liu, X., Shao, W., Zheng, Y., et al. (2017) GSH-Responsive supramolecular Nanoparticles Constructed by β-D-Galactose- Modified Pillararene and Camptothecin Prodrug for Targeted Anticancer Drug Delivery. Chemical Communications, 53, 8596-8599.
https://doi.org/10.1039/C7CC04932C - 6. Zhang, Y.H., Zhang, Y.M., Sheng, X., et al. (2020) Enzyme-Responsive Fluorescent Camptothecin Prodrug/Poly- saccharide Supramolecular Assembly for Targeted Cellular Imaging and in Situ Controlled Drug Release. Chemical Communications, 56, 1042-1045.
https://doi.org/10.1039/C9CC08491F - 7. Whang, C.H., Yoo, E., Hur, S.K., et al. (2018) A Highly GSH-Sensitive SN-38 Prodrug with an “OFF-to-ON” Fluorescence Switch as a Bifunctional Anticancer Agent. Chemical Communications, 54, 9031-9034.
https://doi.org/10.1039/C8CC05010D - 8. Sun, G., He, Z., Hao, M., et al. (2019) Bifunctional Supramolecular Prodrug Vesicles Constructed from a Camptothecin Derivative with a Water-Soluble Pillararene for Cancer Diagnosis and Therapy. Chemical Communications, 55, 10892-10895.
https://doi.org/10.1039/C9CC05859A - 9. Zou, H., Liu, J., Li, Y., et al. (2018) Cucurbituril-Based Polymers and Polymer Materials. Small, 14, 1802234- 1802252.
https://doi.org/10.1002/smll.201802234 - 10. Ding, Y.F., Wei, J., Li, S., et al. (2019) Host-Guest Interactions Initiated Supramolecular Chitosan Nanogels for Selective Intracellular Drug Delivery. ACS Applied Materials & Interfaces, 11, 28665-28670.
https://doi.org/10.1021/acsami.9b09059 - 11. Feng, W., Jin, M., Yang, K., et al. (2018) Supramolecular Delivery Systems Based on Pillararenes. Chemical Communications, 54, 13626-13640.
https://doi.org/10.1039/C8CC08252A - 12. Zhang, Y.M., Zhang, N.Y., Xiao, K., et al. (2018) Photo-Controlled Reversible Microtubule Assembly Mediated by Paclitaxel-Modified Cyclodextrin. Angewandte Chemie International Edition, 57, 8649-8653.
https://doi.org/10.1002/anie.201804620 - 13. Wu, D., Li, Y., Shen, J., et al. (2018) Supramolecular Chemotherapeutic Drug Constructed from Pillararene-Based Supramolecular Amphiphile. Chemical Communications, 54, 8198-8201.
https://doi.org/10.1039/C8CC04334E - 14. Yu, G., Yu, W., Mao, Z., et al. (2015) A Pillararene-Based Ternary Drug-Delivery System with Photocontrolled Anticancer Drug Release. Small, 11, 919-925.
https://doi.org/10.1002/smll.201402236 - 15. Luo, C., Sun, J., Liu, D., et al. (2016) Self-Assembled Redox Dual-Responsive Prodrug-Nanosystem Formed by Single Thioether-Bridged Paclitaxel-Fatty Acid Conjugate for Cancer Chemotherapy. Nano Letters, 16, 5401-5408.
https://doi.org/10.1021/acs.nanolett.6b01632 - 16. Cai, Y., Shen, H., Zhan, J., et al. (2017) Supramolecular “Trojan Horse” for Nuclear Delivery of Dual Anticancer Drugs. Journal of the American Chemical Society, 139, 2876-2879.
https://doi.org/10.1021/jacs.6b12322 - 17. Mori, M., Fujikawa, Y., Kikkawa, M., et al. (2019) A Highly Selective Fluorogenic Substratefor Imaging Glutathione S-Transferase P1: Development and Cellular Applicability in Epigenetic Studies. Chemical Communications, 55, 8122- 8125.
https://doi.org/10.1039/C9CC03064F - 18. Zhang, B., Ge, C., Yao, J., et al. (2015) Selective Selenol Fluorescent Probes: Design, Synthesis, Structural Determinants, and Biological Applications. Journal of the American Chemical Society, 137, 757-769.
https://doi.org/10.1021/ja5099676
NOTES
*通讯作者。