Journal of Physiology Studies
Vol.04 No.01(2016), Article ID:17262,9
pages
10.12677/JPS.2016.41001
Pathogenesis and Treatment of Chronic Hyperuricemic Nephropathy
Xiaolu Meng1, Xin Li2, Baoxue Yang1, Hong Zhou1*
1State Key Laboratory of Natural and Biomimetic Drugs, Department of Pharmacology, School of Basic Medical Sciences, Peking University, Beijing
2The Affiliated Hospital of Qingdao University, Qingdao Shandong

Received: Mar. 9th, 2016; accepted: Mar. 27th, 2016; published: Mar. 30th, 2016
Copyright © 2016 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



ABSTRACT
Large numbers of epidemiological and clinical studies in recent years have accumulated that hyperuricemia may be strongly linked with many metabolic diseases, including hypertension, cardiac-cerebral vascular disease, diabetes and metabolic syndrome. It has been regarded as a severe disease threatening human health and an independent risk factor in the progression of kidney diseases as well. As the end product of purine metabolism, uric acid can induce renal injury through multiple mechanisms, such as inflammation, renal fibrosis, oxidative stress and epithelial-to-mesenchymal transition. To further study the pathogenesis of hyperuricemia nephropathy and potential targets involved, we mainly discuss the physiopathologic mechanism and medical treatment of hyperuricemia in this review.
Keywords:Gout, Hyperuricemia, Chronic Kidney Diseases (CKD), Medical Treatment

高尿酸血症性肾病的发病机制及药物治疗
孟晓璐1,李欣2,杨宝学1,周虹1*
1北京大学医学部基础医学院药理学系,天然药物及仿生药物国家重点实验室,北京
2青岛大学附属医院,山东 青岛

收稿日期:2016年3月9日;录用日期:2016年3月27日;发布日期:2016年3月30日

摘 要
近年来大规模流行病学研究发现,高尿酸血症与高血压、心脑血管疾病、糖尿病、代谢综合征等疾病的发生和发展密切相关,已成为威胁人类健康的严重代谢性疾病,也是肾脏疾病进展的独立危险因素。尿酸作为嘌呤代谢的终末产物,可以通过多种分子机制导致肾脏损伤,包括诱导炎症反应和肾脏间质纤维化,激活氧化应激,诱导肾小管上皮细胞向间质细胞转化(epithelial-to-mesenchymal transition, EMT)等。为了深入研究高尿酸血症性肾脏疾病的发病机制,寻找新的药物治疗靶点,本文对高尿酸血症性肾病发生发展的病理生理机制及其治疗药物的研究进展进行综述。
关键词 :痛风,高尿酸血症,慢性肾脏疾病,药物治疗

1. 引言
尿酸是嘌呤代谢的终末产物。由于人体血浆中尿酸盐在7 mg/dL时达到饱和,所以人血浆尿酸盐的浓度超过7 mg/dL通常称为高尿酸血症。在大多数哺乳动物体内,由嘌呤代谢生成的尿酸可以由肝脏中的尿酸酶降解为尿囊素随尿液排出。然而,灵长类动物的尿酸酶基因在进化的过程中发生了突变,因而人体内并无尿酸酶表达,从而使人体的血尿酸水平相对较高 [1] 。随着生活水平的提高,高嘌呤食物摄入的增加、嘌呤代谢紊乱导致尿酸生成过多或肾脏排泄尿酸减少,均易使血尿酸升高,导致高尿酸血症,进而引起肾脏损伤。目前,高尿酸血症的患病率日益增加,高尿酸血症不仅可导致痛风,还可引起明显的肾脏损伤,应引起高度的重视。因此,控制高尿酸血症是防治高尿酸血症性肾病和肾功能减退的重要措施。本文对高尿酸血症性肾病的发病机制及药物治疗进展进行综述。
2. 高尿酸血症与慢性肾脏疾病
慢性肾脏疾病(chronic kidney disease, CKD)是指出现肾脏结构或功能异常超过3个月,并影响健康状况的肾脏疾病 [2] 。目前,CKD已经成为世界范围内危害人类健康的重要疾病,并受到广泛关注。据报道,美国有超过13%的人群患有CKD,超过了患糖尿病的人数 [3] 。其中,在20岁以上的人群里,有16.8%的患病率;而在65岁以上的人群中,有高达47.3%的患病率 [4] 。我国的最新调查显示18岁以上的成年人中CKD患病率为10.8%,而且以平均每年11%的速度增长。因此,CKD已成为全球性的公共卫生问题。
改善全球肾脏病预后组织(Kidney Disease: Improving Global Outcomes, KDIGO)发布的最新CKD治疗指南将慢性肾病分为四个时期 [5] 。由于肾小球滤过率(glomerular filtration rate, GFR)下降与尿蛋白含量增加分别是影响肾功能的独立危险因素,因此将GFR与蛋白尿结合起来,共同作为评估肾功能的标准。高尿酸血症作为一个独立的致病因素对CKD的各个时期均有不同程度的危害。一项涉及13项实验、190718名患者的meta分析发现高尿酸血症是导致CKD发生的独立致病因素 [6] 。Kohagura K等对167名日本CKD患者的活体检查发现,当患者血尿酸水平高于7 mg/dL时,高尿酸会直接加重肾脏小动脉损伤 [7] 。Magdalena Madero等对838名CKD3-4期患者的研究发现,患者体内血尿酸水平每升高1 mg/dL,全因死亡率升高17%。说明尿酸是CKD3-4期的独立危险因素 [8] 。Yejin Mok等对14939名韩国CKD患者的研究表明,CKD 2-4期患者体内血尿酸水平与慢性肾损伤程度成正相关 [9] 。Gianne Bellomo对900名健康成年人的血液检测研究发现,尿酸水平与估测肾小球滤过率(estimated glomerular filtration rate, eGFR)成正相关 [10] 。因此,高尿酸血症与CKD的发生、发展密切相关。
3. 高尿酸血症引起慢性肾脏损伤的分子机制
早期研究认为,高尿酸所造成的肾脏损伤来源于尿酸盐结晶的直接损伤。近年来,大量的临床研究和流行病学表明,高尿酸可以通过多种分子机制导致肾脏损伤,包括引起肾小球系膜细胞损伤,诱导炎症反应和肾脏间质纤维化,激活氧化应激,诱导EMT等。尿酸盐在肾小球自由滤过后,由位于近曲小管的尿酸盐转运子重吸收和再分泌;且当尿酸过饱和时,尿酸主要结晶在肾小管和集合管中,肾小管基底膜细胞剥落;又由于大量的线粒体分布在近曲小管上皮细胞,使其易受活性氧等因素的侵害。因此,尿酸对肾脏的损伤主要涉及肾小管细胞,进而引发肾小管间质病变,最终导致不可逆的肾功能损害,甚至出现终末期肾病(end-stage renal disease, ESRD)或肾功能衰竭。现将近年来研究中涉及的相关机制总结如下。
3.1. 炎症反应与纤维化
高尿酸可以通过激活多条信号通路导致肾脏血管和肾小管细胞损伤,从而诱导炎症反应的发生 [11] 。而炎性细胞因子的表达可以激活促纤维化因子,使基质蛋白合成增多,细胞外基质(extracellular matrix, ECM)沉积,导致肾小管间质纤维化。肾小管间质纤维化是CKD进展至ESRD的最终通路。CKD尽管病因不同,但最终都将归结为肾间质纤维化这一病理过程。因此,抑制炎症反应及纤维化进程具有重要的临床意义。
目前已有大量研究证实丝裂原激活蛋白激酶(mitogen-activated protein kinase, MAPK)信号转导系统在肾脏炎症和纤维化过程中有重要作用。p38 MAPK信号通路是MAPK通路的分支之一,参与了细胞凋亡、炎性反应及纤维化的发生和发展过程。尿酸可以激活PKC/MAPK/cPLA2通路,使环氧酶-2 (cyclooxygenase-2, COX-2)表达增加,诱导血管平滑肌细胞和肾小管上皮细胞产生单核细胞趋化蛋白(monocyte chemoattractant protein1, MCP-1),上调血小板源性生长因子(platelet-derived growth factor, PDGF)。MCP-1和PDGF可以直接引起血管病变和肾脏损害 [12] [13] 。此外,胞外信号调节激酶(extracellular signal-re- gulated kinase, ERK)作为MAPK家族的一员,在尿酸性肾病的发展过程中也十分关键。尿酸通过激活NADPH/ROS/ERK1/2信号通路诱导血管内皮细胞和平滑肌细胞的C-反应蛋白(C-reactive protein, CRP)表达上调,减少NO的合成,导致入球小动脉的血管平滑肌增生 [14] 。
核转录因子(nuclear factor kappa B, NF-κB)是炎症的核心调节因子,与尿酸性肾损伤密切相关。尿酸通过激活NF-κB信号通路促进肾脏肿瘤坏死因子α (tumor necrosis factor-α, TNF-α)和白介素(interleukins, ILs)的基因表达,诱导肾小管上皮细胞产生细胞间粘附分子,加剧肾间质的炎症反应,并上调肾小管上皮细胞赖氨酰氧化酶、细胞间黏附分子1 (intercellular adhesion molecule 1, ICAM-1)和血管细胞粘附分子1 (vascular cell adhesion molecule 1, VCAM-1)的表达,使ECM合成增加,导致间质纤维化 [15] 。
此外,转化生长因子(transforming growth factor-β1, TGF-β1)信号通路对肾小球萎缩和肾小管间质纤维化等病变也起到重要的调节作用。TGF-β1与TGF-β1受体结合后,激活Smad2,Smad3,磷酸化的Smad2和Smad3与Smad4结合后一起转位入核,通过核转录激活TGF-β1下游的靶基因,促进胶原I、II、IV及纤维连接蛋白(fibronectin, FN)等多种ECM成分的产生 [16] 。同时,TGF-β1还可以通过反式激活细胞表皮生长因子受体(epidermal growth factor receptor, EGFR),调节促纤维化过程的产生,并激活其下游的信号通路,如ERK1/2通路,PI3K/Akt信号通路,JAK/STAT信号通路等。
除了以上三条调节炎症和纤维化的经典信号通路,近年来又有一些新的发现。EGFR作为ErbB家族成员之一,具有酪氨酸激酶活性,是一种重要的跨膜受体。EGFR的过度表达或突变或者与之结合的配体的高度表达,都会导致肿瘤的产生,如非小细胞肺癌、乳腺癌、宫颈癌、胃癌等。因此,在以往的研究中EGFR抑制剂主要用于治疗癌症。而最近的研究发现,EGFR与肾脏的炎症及纤维化过程密切相关。EGFR除了受TGF-β1的调控,在血管紧张素II、内皮素-1或氧化应激等环境因素的刺激下,也可诱导EGFR的反式激活。在高尿酸血症大鼠模型中,EGFR的磷酸化水平明显升高,并导致严重的肾小球萎缩和肾间质纤维化。给予EGFR抑制剂后,肾脏损伤明显减轻,尿中微蛋白含量降低,并且可以抑制TGF-β1和NF-κB信号通路,减少促纤维化细胞因子的表达。此外,EGFR抑制剂还可使黄嘌呤氧化酶(xanthine oxidase, XOD)表达水平降低,减少尿酸的合成;促进有机阴离子转运子1 (organic anion transporter1, OAT1)和有机阴离子转运子3 (organic anion transporter3, OAT3)的表达,促进尿酸排出 [17] 。
G蛋白偶联的雌激素受体(G protein coupled estrogen receptor, GPER)是迄今发现的最重要的雌激素膜性受体。最近发现GPER的激活可以抑制TGF-β1激活的Smad2和Smad3的磷酸化以及Smad4复合体的形成,由此抑制Smad信号通路,减少下游Ⅳ型胶原蛋白和纤连蛋白的表达量,阻滞系膜基质的形成,调控系膜细胞迁移,从而减轻炎症及纤维化水平 [18] 。
另外,Jacobien C等发现,肝细胞核因子1β (hepatocyte nuclear factor 1β, HNF1β)可能与高尿酸血症性肾病相关。HNF1β可以调控尿调节素(UMOD)基因的转录,而尿调节可以调控尿酸转运子。因此,HNF1β突变的患者大多数都患有高尿酸血症和早发性痛风 [19] 。
XOD作为体内合成尿酸的关键酶,一直以来人们认为嘌呤代谢紊乱是导致XOD活性增加,合成过量尿酸的唯一因素。而Nomura J等在体外实验中发现,XOD可被多种炎症刺激因素上调,包括脂多糖、缺氧和促炎性细胞因子,进而形成过量的ROS,下调丝裂原活化蛋白激酶-1 (mitogen-activated protein kinasephosphatase-1, MKP-1),从而调控脂多糖诱导的应激活化蛋白激酶(c-Jun N-terminal kinase, JNK)磷酸化,导致MCP-1在巨噬细胞中表达,造成组织损伤 [20] 。
3.2. 氧化应激与内皮损伤
氧化应激是指机体促氧化与抗氧化之间失衡导致的组织损伤,主要涉及活性氮(reactive nitrogen species, RNS)和活性氧(reactive oxygen species, ROS)。RNS包括NO、ONOO·、HOONO等具有高度氧化活性的自由基和硝基类化合物。研究发现,尿酸可以引起血管内皮细胞NADPH氧化酶的NOX-4亚基表达增加,血管紧张素II表达增加,肾脏血管内皮细胞内皮素-1 (endothelin-1, ET-1)的生成,抑制致密斑中一氧化氮合酶(nitric oxide synthase, NOS)的生成,NO生物利用度降低,尿液中NO代谢物减少(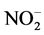 /
/ )。而NO作为最强的内源性血管舒张因子,被认为是血管内皮功能紊乱最重要、最早期的表现。因此,NO活性降低导致内皮损伤,同时损害线粒体功能 [21] 。此外,体外给予胰岛β细胞尿酸刺激,可促进IκBα的磷酸化水平,激活NF-κB信号通路,上调诱导型一氧化氮合酶(inducible nitric oxide synthase, iNOS)的表达,产生过量的NO。即尿酸可以通过NF-κB-iNOS-NO通路直接造成胰岛β细胞损伤 [22] 。
)。而NO作为最强的内源性血管舒张因子,被认为是血管内皮功能紊乱最重要、最早期的表现。因此,NO活性降低导致内皮损伤,同时损害线粒体功能 [21] 。此外,体外给予胰岛β细胞尿酸刺激,可促进IκBα的磷酸化水平,激活NF-κB信号通路,上调诱导型一氧化氮合酶(inducible nitric oxide synthase, iNOS)的表达,产生过量的NO。即尿酸可以通过NF-κB-iNOS-NO通路直接造成胰岛β细胞损伤 [22] 。
ROS是体内氧化应激的主要来源,包括超氧阴离子(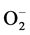 )、羟自由基(OH)、氢过氧自由基、过氧化氢和单电键氧等,它们对正常的肾脏组织均可造成损伤。XOD在合成尿酸的过程中,将次黄嘌呤氧化为黄嘌呤,进而氧化为尿酸,同时产生超氧阴离子。过量的超氧阴离子会造成慢性肾脏损伤、慢性心脏损伤以及缺血再灌注损伤等 [23] 。Sen S等发现,给予超氧阴离子清除剂印度药用植物Meyna spinosa叶子提取物可以减轻由尿酸造成的肾脏损伤 [24] 。
)、羟自由基(OH)、氢过氧自由基、过氧化氢和单电键氧等,它们对正常的肾脏组织均可造成损伤。XOD在合成尿酸的过程中,将次黄嘌呤氧化为黄嘌呤,进而氧化为尿酸,同时产生超氧阴离子。过量的超氧阴离子会造成慢性肾脏损伤、慢性心脏损伤以及缺血再灌注损伤等 [23] 。Sen S等发现,给予超氧阴离子清除剂印度药用植物Meyna spinosa叶子提取物可以减轻由尿酸造成的肾脏损伤 [24] 。
3.3. 肾小管上皮细胞表型转化
EMT指上皮细胞通过特定程序转化为具有间质表型细胞的生物学过程。在这一过程中,上皮细胞失去细胞极性及与基底膜连接等上皮表型,获得了较高的迁移与侵袭能力、抗凋亡等间质表型。这一症状预示着肾脏纤维化的产生。研究发现,尿酸可以抑制E-钙黏蛋白(E-cadherin) mRNA的转录,并引起下游Snail、Slug和Twist转录因子的过表达,从而抑制E-cadherin的合成;同时,通过泛素化作用使E-cadherin降解。E-cadherin在上皮细胞中介导细胞连接,参与细胞分化并抑制细胞迁移,因此,E-cadherin表达下调会使细胞相互分离,形成成纤维细胞样的细胞 [25] 。此外,尿酸可以促进α-平滑肌肌动蛋白(α-smooth muscle actin, α-SMA)的表达。而α-SMA是肌成纤维细胞的标志蛋白,肌成纤维细胞具有活跃的增殖和分泌胶原的功能,引起ECM沉积,肾小管萎缩,肾脏纤维化。同时,转化生长因子β (transforming growth factor-β, TGF-β)也可以通过引起Snail、Slug和Twist转录因子的过表达而抑制E-cadherin的合成 [26] 。并且由于这一过程的可逆性,EMT被认为是治疗肾损伤的重要靶点。
4. 高尿酸性肾病的药物治疗
4.1. XOD抑制剂
机体内嘌呤经过一系列代谢过程水解成次黄嘌呤,并在XOD的催化下逐步氧化为黄嘌呤和尿酸。因此,抑制XOD活性可抑制尿酸的生成。
别嘌呤醇是目前应用最为广泛的降尿酸药物。别嘌呤醇是一种嘌呤类化合物,在肝脏中可代谢成为氧嘌呤醇,其与XOD的亲和力均强于次黄嘌呤和黄嘌呤,因此它可以竞争性抑制XOD的活性,从而抑制次黄嘌呤向黄嘌呤、黄嘌呤向尿酸的转化,即抑制尿酸合成。但是它也存在一定的局限性。在采用别嘌呤醇的临床治疗中,许多病患并未达到使血尿酸降至6 mg/dL的目标。Stamp等提出了导致这种抵抗作用的四种可能性机制,包括别嘌呤醇向氧嘌呤醇的转化率降低、肾脏对氧嘌呤醇的排泄率增高、XOD结构或功能不正常和多种药物间存在相互作用 [27] 。同时,也有不少研究表明别嘌呤醇可能会引起超敏反应综合征 [28] [29] 。并且在用药上需注意减少初始剂量,并循序渐进给药,以达到最佳治疗效果。
非布司他是一种非嘌呤类XOD抑制剂,并可以同时抑制XOD的氧化形式和还原形式,而别嘌呤醇只可与XOD的还原形式结合 [30] 。非布司他在肝脏内经过氧化反应和醛糖酸化反应代谢,并可由尿液和粪便两种途径排出体外,因此对有肾脏损伤的患者而言有更好的疗效和耐受性 [31] ,并且对轻度或中度肾损伤患者无需调整剂量 [32] 。非布司他对嘌呤代谢和嘧啶代谢过程中的其他酶无相互作用,因此具有较好的专一性和选择性 [33] 。在动物实验中,非布司他的降尿酸效率明显高于别嘌呤醇。同时,三期临床实验也证实了非布司他在治疗尿酸性肾病上的有效性和高耐受性 [34] [35] 。2012年美国风湿病学会(American college of rheumatology, ACR)痛风治疗指南将别嘌醇和非布司他作为降尿酸的首选药物。
除了以上两种经典药物,最近有研究发现1-羟基/甲氧基-4-甲基-2-苯基-1H-咪唑-5羧基的羧酸衍生物有较非布司他更好的XOD抑制作用 [36] 。Yoon S等对一种新型的非嘌呤类XOD抑制剂LC350189进行了药代动力学、药效学以及耐药性研究,发现LC350189在10~800 mg范围内均可耐受,并有良好的降尿酸效果 [37] 。Zafar H等对2-芳基-喹唑啉-4(3H)-酮类化合物的研究表明,其中一些化合物对XOD有较好的抑制作用 [38] 。BCX4208是一种嘌呤核苷酸磷酸酶(purine nucleoside phosphorylase, PNP)抑制剂,嘌呤核苷酸磷酸酶在嘌呤代谢中处于XOD的上游,催化生成黄嘌呤。BCX4208目前已处于二期临床试验阶段 [39] 。
此外,目前许多研究证实了一些中药成分也可抑制XOD,防治高尿酸血症。体外实验已证实,黄酮、生物碱、挥发油、酚类、丹宁酸、环烯醚萜苷、香豆素等化合物可抑制XOD的活性。同时,新的中药成分如绿茶多酚可以有效降低血尿酸水平并呈剂量依赖关系,并可以抑制肝脏中XOD的活性及肾脏中URAT1的表达,同时上调有机阴离子转运蛋白1和有机阴离子转运蛋白3的表达 [40] 。Chen WJ等对二妙丸系列类方(二妙丸、三妙丸、四妙丸及加味四妙丸)进行研究,发现可以有效抑制XOD活性,并缓解肾小球萎缩、血管循环紊乱、炎症细胞渗入及肾间质纤维化 [41] 。有文献报道了对苦舌草的研究,从中分离纯化出两种苯乙醇苷类化合物苯丙素苷和毛蕊花苷,三种黄酮类化合物6-羟基木犀草素、6-羟基木犀草素-7-O-糖苷和过江藤素。它们均可抑制XOD活性,值得进一步研究 [42] 。绿原酸是一种多酚类化合物,它具有对XOD的抑制作用,并可以抑制促炎症因子白细胞介素-1β (interleukin-1β, IL-1β)、白细胞介素-6 (interleukin-6, IL-6)和肿瘤坏死因子-α (tumor necrosis factor-α, TNF-α)的表达 [43] 。
4.2. 尿酸转运子及葡萄糖转运子抑制剂
尿酸由肾脏和肠道排出,其中大约75%的尿酸是经过肾脏进行代谢的。尿酸合成后,通过定位于近端肾小管上皮细胞基底侧膜的有机阴离子转运子1 (organic anion transporters-1, OAT1)和有机阴离子转运子3 (organic anion transporters-3, OAT3),从血液进入肾近曲小管细胞,再分泌入管腔。同时,尿酸转运子1 (urate transporter 1, URAT1)表达于近曲小管细胞顶膜,参与尿酸盐的重吸收。有研究表明,血管紧张素Ⅱ受体拮抗剂洛沙坦可以通过抑制URAT1,减少尿酸盐的重吸收 [44] 。给予1342名患者洛沙坦6个月后,患者的血尿酸水平显著降低,并且血尿酸水平每降低0.5 mg/dL,肾脏病变就减少6% [45] 。
SGLT2是一种高容量、低亲和力的葡萄糖转运子,主要表达于肾脏的近曲小管,负责90%的葡萄糖重吸收。近期的研究发现,抑制SGLT2可以减少血尿酸的水平。其机制可能是由于抑制了SGLT2后,尿中葡萄糖含量增高,从而导致更多的尿酸交换到肾小管腔,即血中尿酸转移到尿中含量增多,因此降低了血尿酸水平 [46] 。试验发现,20%~30%的高尿酸患者在服用SGLT2抑制剂坎格列净(Canagliflozin) 26周后血尿酸水平恢复正常 [47] 。并且,给予2型糖尿病患者SGLT2抑制剂依帕列净24周后,其血尿酸水平也明显降低 [48] 。
4.3. 氧化应激及炎症因子抑制剂
研究发现苯溴马隆可以直接清除超氧阴离子自由基,并在内皮细胞中抑制由血管紧张素Ⅱ或尿酸刺激产生的ROS,从而缓解尿酸性肾损伤 [49] 。另外,一些天然化合物,如虎杖甙,在高尿酸小鼠模型中被证实有抗氧化的作用,同时可以下调NF-κB、COX-2及iNOS的表达,抑制炎性因子TNF-α,PGE2和IL-1β的活性 [50] 。
4.4. 尿酸氧化酶
尿酸氧化酶(尿酸酶;EC1.7.3.3;UOX)是一种在嘌呤代谢途径中催化尿酸氧化生成尿囊素的酶。在灵长类动物漫长的进化过程中,人科动物尿酸氧化酶基因突变失活,成为假基因 [51] 。因此,可以通过给予尿酸氧化酶来下调尿酸水平,缓解高尿酸血症。
4.5. 药物联合应用治疗高尿酸血症性肾病
为了进一步减轻或预防药物的不良反应及增强疗效,目前许多研究者开始尝试两种药物结合用药。近期发现非布司他40或80 mg/d和RDEA 594 400或600 mg/d联合用药,降尿酸效果比二者单独用药更好,且耐受性良好 [52] 。Azevedo认为苯溴马隆和别嘌呤醇联合使用,可以达到更好的降尿酸效果,对于单独用药无法达到治疗标准的患者来说,是一个很好的选择 [53] 。
5. 结语
随着临床和实验研究数据的不断积累,人们对高尿酸血症和慢性肾病的了解日益深入,对二者的联系及致病机制也有了较为深刻的认识。但是,慢性高尿酸血症性肾病依然是世界范围内的一种较为常见的疾病,亟需开发出疗效更好、毒副作用更小的药物。近年来,中医药对慢性尿酸血症性肾病的治疗取得了较大进步,其多靶点的特性得以开发。而开发联合用药的方式,发挥不同药物的优势实现互补并减轻毒副作用,对于治疗慢性高尿酸血症性肾病具有重要意义。
基金项目
国家自然科学基金资助项目(No. 81370783)。
文章引用
孟晓璐,李 欣,杨宝学,周 虹. 高尿酸血症性肾病的发病机制及药物治疗
Pathogenesis and Treatment of Chronic Hyperuricemic Nephropathy[J]. 生理学研究, 2016, 04(01): 1-9. http://dx.doi.org/10.12677/JPS.2016.41001
参考文献 (References)
- 1. Mazzali, M., Hughes, J., Kim, Y.G., Jefferson, J.A., Kang, D.H., Gordon, K.L., et al. (2001) Elevated Uric Acid Increases Blood Pressure in the Rat by a Novel Crystal-Independent Mechanism. Hypertension, 38, 1101-1106. http://dx.doi.org/10.1161/hy1101.092839
- 2. Stevens, P.E. and Levin, A. (2013) Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Annals of Internal Medicine, 158, 825-830. http://dx.doi.org/10.7326/0003-4819-158-11-201306040-00007
- 3. Coresh, J., Selvin, E., Stevens, L.A., Manzi, J., Kusek, J.W., Eggers, P., et al. (2007) Prevalence of Chronic Kidney Disease in the United States. JAMA, 298, 2038-2047. http://dx.doi.org/10.1001/jama.298.17.2038
- 4. Patel, N., Golzy, M., Nainani, N., Nader, N.D., Carter, R.L., Lohr, J.W., et al. (2015) Prevalence of Various Comorbidities among Veterans with Chronic Kidney Disease and Its Comparison with Other Datasets. Renal Failure, 1-5.
- 5. Inker, L.A., Astor, B.C., Fox, C.H., Isakova, T., Lash, J.P., Peralta, C.A., et al. (2014) KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for the Evaluation and Management of CKD. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation, 63, 713-735. http://dx.doi.org/10.1053/j.ajkd.2014.01.416
- 6. Li, L., Yang, C., Zhao, Y., Zeng, X., Liu, F. and Fu, P. (2014) Is Hyperuricemia an Independent Risk Factor for New-Onset Chronic Kidney Disease?: A Systematic Review and Meta-Analysis Based on Observational Cohort Studies. BMC Nephrology, 15, 122. http://dx.doi.org/10.1186/1471-2369-15-122
- 7. Kohagura, K., Kochi, M., Miyagi, T., Kinjyo, T., Maehara, Y., Nagahama, K., et al. (2013) An Association between Uric Acid Levels and Renal Arteriolopathy in Chronic Kidney Disease: A Biopsy-Based Study. Hypertension Research: Official Journal of the Japanese Society of Hypertension, 36, 43-49. http://dx.doi.org/10.1038/hr.2012.135
- 8. Madero, M., Sarnak, M.J., Wang, X., Greene, T., Beck, G.J., Kusek, J.W., et al. (2009) Uric Acid and Long-Term Outcomes in CKD. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation, 53, 796-803. http://dx.doi.org/10.1053/j.ajkd.2008.12.021
- 9. Mok, Y., Lee, S.J., Kim, M.S., Cui, W., Moon, Y.M. and Jee, S.H. (2012) Serum Uric Acid and Chronic Kidney Disease: The Severance Cohort study. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association—European Renal Association, 27, 1831-1835. http://dx.doi.org/10.1093/ndt/gfr530
- 10. Bellomo, G., Venanzi, S., Verdura, C., Saronio, P., Esposito, A. and Timio, M. (2010) Association of Uric Acid with Change in Kidney Function in Healthy Normotensive Individuals. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation, 56, 264-272. http://dx.doi.org/10.1053/j.ajkd.2010.01.019
- 11. Wang, Y. and Bao, X. (2013) Effects of Uric Acid on Endothelial Dysfunction in Early Chronic Kidney Disease and Its Mechanisms. European Journal of Medical Research, 18, 26. http://dx.doi.org/10.1186/2047-783X-18-26
- 12. Pal, P.B., Sinha, K. and Sil, P.C. (2014) Mangiferin Attenuates Diabetic Nephropathy by Inhibiting Oxidative Stress Mediated Signaling Cascade, TNFα Related and Mitochondrial Dependent Apoptotic Pathways in Streptozotocin-In- duced Diabetic Rats. PLoS ONE, 9, e107220. http://dx.doi.org/10.1371/journal.pone.0107220
- 13. Liu, C.M., Sun, Y.Z., Sun, J.M., Ma, J.Q. and Cheng, C. (2012) Protective Role of Quercetin against Lead-Induced Inflammatory Response in Rat Kidney through the ROS-Mediated MAPKs and NF-κB Pathway. Biochimica et Biophysica Acta (BBA)-General Subjects, 1820, 1693-1703. http://dx.doi.org/10.1016/j.bbagen.2012.06.011
- 14. Zhuang, Y., Feng, Q., Ding, G., Zhao, M., Che, R., Bai, M., et al. (2014) Activation of ERK1/2 by NADPH Oxidase- Originated Reactive Oxygen Species Mediates Uric Acid-Induced Mesangial Cell Proliferation. American Journal of Physiology. Renal Physiology, 307, F396-F406. http://dx.doi.org/10.1152/ajprenal.00565.2013
- 15. Zhou, Y., Fang, L., Jiang, L., Wen, P., Cao, H., He, W., et al. (2012) Uric Acid Induces Renal Inflammation via Activating Tubular NF-κB Signaling Pathway. PLoS ONE, 7, e39738. http://dx.doi.org/10.1371/journal.pone.0039738
- 16. Lyngdoh, T., Marques-Vidal, P., Paccaud, F., et al. (2011) Elevated Serum Uric Acid Is Associated with High Circulating Inflammatory Cytokines in the Popula-tion-Based Colaus Study. PLoS One, 6, e19901. http://dx.doi.org/10.1371/journal.pone.0019901
- 17. Liu, N., Wang, L., Yang, T., Xiong, C., Xu, L., Shi, Y., et al. (2015) EGF Receptor Inhibition Alleviates Hyperuricemic Nephropathy. Journal of the American Society of Nephrology, 26, 2716-2729. http://dx.doi.org/10.1681/ASN.2014080793
- 18. Li, Y.C., Ding, X.S., Li, H.M., Zhang, Y. and Bao, J. (2014) Role of G Protein-Coupled Estrogen Receptor 1 in Modulating Transforming Growth Factor-Beta Stimulated Mesangial Cell Extracellular Matrix Synthesis and Migration. Molecular and Cellular Endocrinology, 391, 50-59. http://dx.doi.org/10.1016/j.mce.2014.04.014
- 19. Verhave, J.C., Bech, A.P., Wetzels, J.F. and Nijenhuis, T. (2015) Hepatocyte Nuclear Factor 1β-Associated Kidney Disease: More than Renal Cysts and Diabetes. Journal of the American Society of Nephrology, 27, 345-353. http://dx.doi.org/10.1681/ASN.2015050544
- 20. Nomura, J., Busso, N., Ives, A., Tsujimoto, S., Tamura, M., So, A., et al. (2013) Febuxostat, an Inhibitor of Xanthine Oxidase, Suppresses Lipopolysaccharide-Induced MCP-1 Production via MAPK Phosphatase-1-Mediated Inactivation of JNK. PLoS ONE, 8, e75527. http://dx.doi.org/10.1371/journal.pone.0075527
- 21. Sanchez-Lozada, L.G., Soto, V., Tapia, E., Avila-Casado, C., Sautin, Y.Y., Nakagawa, T., et al. (2008) Role of Oxidative Stress in the Renal Abnormalities Induced by Experimental Hyperuricemia. American Journal of Physiology. Renal Physiology, 295, F1134-F1141. http://dx.doi.org/10.1152/ajprenal.00104.2008
- 22. Jia, L., Xing, J., Ding, Y., Shen, Y., Shi, X., Ren, W., et al. (2013) Hyperuricemia Causes Pancreatic Beta-Cell Death and Dysfunction through NF-κB Signaling Pathway. PLoS ONE, 8, e78284. http://dx.doi.org/10.1371/journal.pone.0078284
- 23. Sugahara, S., Ueda, Y., Fukuhara, K., Kamamuta, Y., Matsuda, Y., Murata, T., et al. (2015) Antioxidant Effects of Herbal Tea Leaves from Yacon (Smallanthus sonchifolius) on Multiple Free Radical and Reducing Power Assays, Especially on Different Superoxide Anion Radical Generation Systems. Journal of Food Science, 80, C2420-C2429. http://dx.doi.org/10.1111/1750-3841.13092
- 24. Sen, S., De, B., Devanna, N. and Chakraborty, R. (2013) Total Phenolic, Total Flavonoid Content, and Antioxidant Capacity of the Leaves of Meyna spinosa Roxb., an Indian Medicinal Plant. Chinese Journal of Natural Medicines, 11, 149-157. http://dx.doi.org/10.1016/S1875-5364(13)60042-4
- 25. Ryu, E.S., Kim, M.J., Shin, H.S., Jang, Y.H., Choi, H.S., Jo, I., et al. (2013) Uric Acid-Induced Phenotypic Transition of Renal Tubular Cells as a Novel Mechanism of Chronic Kidney Disease. American Journal of Physiology. Renal Physiology, 304, F471-F480. http://dx.doi.org/10.1152/ajprenal.00560.2012
- 26. Dave, N., Guaita-Esteruelas, S., Gutarra, S., Frias, A., Beltran, M., Peiro, S., et al. (2011) Functional Cooperation between Snail1 and Twist in the Regulation of ZEB1 Expression during Epithelial to Mesenchymal Transition. The Journal of Biological Chemistry, 286, 12024-12032. http://dx.doi.org/10.1074/jbc.M110.168625
- 27. Stamp, L.K., Merriman, T.R., Barclay, M.L., Singh, J.A., Roberts, R.L., Wright, D.F., et al. (2014) Impaired Response or Insufficient Dosage? Examining the Potential Causes of “Inadequate Response” to Allopurinol in the Treatment of Gout. Seminars in Arthritis and Rheumatism, 44, 170-174. http://dx.doi.org/10.1016/j.semarthrit.2014.05.007
- 28. Thurston, M.M., Phillips, B.B. and Bourg, C.A. (2013) Safety and Efficacy of Allopurinol in Chronic Kidney Disease. The Annals of Pharmacotherapy, 47, 1507-1516. http://dx.doi.org/10.1177/1060028013504740
- 29. Lam, M.P., Yeung, C.K. and Cheung, B.M. (2013) Pharmacogenetics of Allopurinol—Making an Old Drug Safer. Journal of Clinical Pharmacology, 53, 675-679. http://dx.doi.org/10.1002/jcph.67
- 30. Hosoya, T., Kimura, K., Itoh, S., Inaba, M., Uchida, S., Tomino, Y., et al. (2014) The Effect of Febuxostat to Prevent a Further Reduction in Renal Function of Patients with Hyperuricemia Who Have Never Had Gout and Are Complicated by Chronic Kidney Disease Stage 3: Study Protocol for a Multicenter Randomized Controlled Study. Trials, 15, 26. http://dx.doi.org/10.1186/1745-6215-15-26
- 31. Garcia-Valladares, I., Khan, T. and Espinoza, L.R. (2011) Efficacy and Safety of Febuxostat in Patients with Hyperuricemia and Gout. Therapeutic Advances in Musculoskeletal Disease, 3, 245-253. http://dx.doi.org/10.1177/1759720X11416405
- 32. Frampton, J.E. (2015) Febuxostat: A Review of Its Use in the Treatment of Hyperuricaemia in Patients with Gout. Drugs, 75, 427-438. http://dx.doi.org/10.1007/s40265-015-0360-7
- 33. Gray, C.L. and Walters-Smith, N.E. (2011) Febuxostat for Treatment of Chronic Gout. American Journal of Health- System Pharmacy, 68, 389-398. http://dx.doi.org/10.2146/ajhp100394
- 34. Sircar, D., Chatterjee, S., Waikhom, R., Golay, V., Raychaudhury, A., Chatterjee, S., et al. (2015) Efficacy of Febuxostat for Slowing the GFR Decline in Patients With CKD and Asymptomatic Hyperuricemia: A 6-Month, Double- Blind, Randomized, Placebo-Controlled Trial. American Journal of Kidney Diseases, 66, 945-950. http://dx.doi.org/10.1053/j.ajkd.2015.05.017
- 35. Xu, S., Liu, X., Ming, J., Chen, S., Wang, Y., Liu, X., et al. (2015) A Phase 3, Multicenter, Randomized, Allopurinol-Controlled Study Assessing the Safety and Efficacy of Oral Febuxostat in Chinese Gout Patients with Hyperuricemia. International Journal of Rheumatic Diseases, 18, 669-678. http://dx.doi.org/10.1111/1756-185X.12648
- 36. Chen, S., Zhang, T., Wang, J., Wang, F., Niu, H., Wu, C., et al. (2015) Synthesis and Evaluation of 1-Hydroxy/me- thoxy-4-methyl-2-phenyl-1H-imidazole-5-carboxylic Acid Derivatives as Non-Purine Xanthine Oxidase Inhibitors. European Journal of Medicinal Chemistry, 103, 343-353. http://dx.doi.org/10.1016/j.ejmech.2015.08.056
- 37. Yoon, S., Shin, D., Lee, H., Jang, I.J. and Yu, K.S. (2015) Pharmacokinetics, Pharmacodynamics, and Tolerability of LC350189, a Novel Xanthine Oxidase Inhibitor, in Healthy Subjects. Drug Design, Development and Therapy, 9, 5033- 5049. http://dx.doi.org/10.2147/DDDT.S86884
- 38. Zafar, H., Saad, S.M., Perveen, S., Arshia, Malik, R., Khan, A., et al. (2015) 2-Arylquinazolin-4(3H)-ones: Inhibitory Activities against Xanthine Oxidase. Medicinal Chemistry, 12, 54-62. http://dx.doi.org/10.2174/1573406410666150807111336
- 39. Jansen, T.L. (2015) Rational Pharmacotherapy (RPT) in Goutology: Define the Serum Uric Acid Target & Treat-to- Target Patient Cohort and Review on Urate Lowering Therapy (ULT) Applying Synthetic Drugs. Joint Bone Spine, 82, 225-229. http://dx.doi.org/10.1016/j.jbspin.2014.02.015
- 40. Chen, G., Tan, M.L., Li, K.K., Leung, P.C. and Ko, C.H. (2015) Green Tea Polyphenols Decreases Uric Acid Level through Xanthine Oxidase and Renal Urate Transporters in Hyperuricemic Mice. Journal of Ethnopharmacology, 175, 14-20. http://dx.doi.org/10.1016/j.jep.2015.08.043
- 41. Chen, W.J., Wu, Y., Xu, C., Liu, S., Wang, W.Z., Song, F.R., et al. (2015) [Study on Therapeutic Effects of Ermiao Pill and Ermiao Pill Categorized Formula in Hyperuricemic Rats Using Spectroscopic Methods]. Spectroscopy and Spectral Analysis, 35, 956-960.
- 42. Cheng, L.C., Murugaiyah, V. and Chan, K.L. (2015) In Vitro Xanthine Oxidase Inhibitory Studies of Lippia nodiflora and Isolated Flavonoids and Phenylethanoid Glycosides as Potential Uric Acid-Lowering Agents. Natural Product Communications, 10, 945-948.
- 43. Meng, Z.Q., Tang, Z.H., Yan, Y.X., Guo, C.R., Cao, L., Ding, G., et al. (2014) Study on the Anti-Gout Activity of Chlorogenic Acid: Improvement on Hyperuricemia and Gouty Inflammation. The American Journal of Chinese Medicine, 42, 1471-1483. http://dx.doi.org/10.1142/S0192415X1450092X
- 44. Mende, C. (2015) Management of Chronic Kidney Disease: The Relationship between Serum Uric Acid and Development of Nephropathy. Advances in Therapy, 32, 1177-1191. http://dx.doi.org/10.1007/s12325-015-0272-7
- 45. Miao, Y., Ottenbros, S.A., Laverman, G.D., Brenner, B.M., Cooper, M.E., Parving, H.H., et al. (2011) Effect of a Reduction in Uric Acid on Renal Outcomes during Losartan Treatment: A Post Hoc Analysis of the Reduction of Endpoints in Non-Insulin-Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension, 58, 2-7. http://dx.doi.org/10.1161/HYPERTENSIONAHA.111.171488
- 46. Chino, Y., Samukawa, Y., Sakai, S., Nakai, Y., Yamaguchi, J., Nakanishi, T., et al. (2014) SGLT2 Inhibitor Lowers Serum Uric Acid through Alteration of Uric Acid Transport Activity in Renal Tubule by Increased Glycosuria. Biopharmaceutics & Drug Disposition, 35, 391-404. http://dx.doi.org/10.1002/bdd.1909
- 47. Davies, M.J., Trujillo, A., Vijapurkar, U., Damaraju, C.V. and Meininger, G. (2015) Effect of Canagliflozin on Serum Uric Acid in Patients with Type 2 Diabetes Mellitus. Diabetes, Obesity & Metabolism, 17, 426-429. http://dx.doi.org/10.1111/dom.12439
- 48. Haring, H.U., Merker, L., Seewaldt-Becker, E., Weimer, M., Meinicke, T., Broedl, U.C., et al. (2014) Empagliflozin as Add-On to Metformin in Patients with Type 2 Diabetes: A 24-Week, Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Care, 37, 1650-1659. http://dx.doi.org/10.2337/dc13-2105
- 49. Kadowaki, D., Sakaguchi, S., Miyamoto, Y., Taguchi, K., Muraya, N., Narita, Y., et al. (2015) Direct Radical Scavenging Activity of Benzbromarone Provides Beneficial Antioxidant Properties for Hyperuricemia Treatment. Biological & Pharmaceutical Bulletin, 38, 487-492. http://dx.doi.org/10.1248/bpb.b14-00514
- 50. Chen, L., Lan, Z., Lin, Q., Mi, X., He, Y., Wei, L., et al. (2013) Polydatin Ameliorates Renal Injury by Attenuating Oxidative Stress-Related Inflammatory Responses in Fructose-Induced Urate Nephropathic Mice. Food and Chemical Toxicology, 52, 28-35. http://dx.doi.org/10.1016/j.fct.2012.10.037
- 51. Dabbagh, F., Ghoshoon, M.B., Hemmati, S., Zamani, M., Mohkam, M. and Ghasemi, Y. (2015) Engineering Human Urate Oxidase: Towards Reactivating It as an Important Therapeutic Enzyme. Current Pharmaceutical Biotechnology, 17, 141-146. http://dx.doi.org/10.2174/1389201016666150907113055
- 52. Fleischmann, R., Kerr, B., Yeh, L.T., Suster, M., Shen, Z., Polvent, E., et al. (2014) Pharmacodynamic, Pharmacokinetic and Tolerability Evaluation of Concomitant Administration of Lesinurad and Febuxostat in Gout Patients with Hyperuricaemia. Rheumatology, 53, 2167-2174. http://dx.doi.org/10.1093/rheumatology/ket487
- 53. Azevedo, V.F., Buiar, P.G., Giovanella, L.H., Severo, C.R. and Carvalho, M. (2014) Allopurinol, Benzbromarone, or a Combination in Treating Patients with Gout: Analysis of a Series of Outpatients. International Journal of Rheumatology, 2014, Article ID: 263720. http://dx.doi.org/10.1155/2014/263720
*通讯作者。