Material Sciences
Vol.07 No.03(2017), Article ID:20496,6
pages
10.12677/MS.2017.73035
Hydrothermal Preparation of La-Doped Bi2WO6 Photocatalyst
Junling Shen, Hongquan Jiang*
Key Laboratory of Design and Synthesis of Functional Materials and Green Catalysis, Colleges of Heilongjiang Province, Harbin Normal University, Harbin Heilongjiang
*通讯作者。

Received: Apr. 16th, 2017; accepted: May 9th, 2017; published: May 12th, 2017

ABSTRACT
The hydrothermal synthesis process of La-doped Bi2WO6 photocatalyst was optimized. The effects of various factors including La-doping amount, initial pH of reactant solution, hydrothermal time, calcination temperature, and calcination time on the photocatalytic activity of La-doped Bi2WO6 were investigated through the photocatalytic degradation of 4-chlorophenol (4-CP) under simulated sunlight irradiation. The results show optimal preparation conditions as follows: La-doping amount is 1.5 wt%, and the initial pH value of reactant solution is 7, and hydrothermal time at 160˚C is 12 h, and calcination temperature is 500˚C, and calcination time is 2 h. The photoactivity of as-prepared La-doped Bi2WO6 was 51%, which is much higher than that of undoped Bi2WO6 prepared under the same conditions (38.4%).
Keywords:Bi2WO6, La-Doping, 4-Chlorophenol, Photocatalysis
La掺杂Bi2WO6光催化剂的水热制备工艺
沈俊玲,姜洪泉*
哈尔滨师范大学功能材料设计合成与绿色催化黑龙江省高校重点实验室,黑龙江 哈尔滨

收稿日期:2017年4月16日;录用日期:2017年5月9日;发布日期:2017年5月12日

摘 要
优化了La掺杂Bi2WO6光催化剂的水热制备工艺。考察了掺杂量、反应液初始pH值、水热时间、焙烧温度及焙烧时间等条件因素对La掺杂Bi2WO6在模拟太阳光下光催化降解4-氯酚(4-CP)性能的影响。结果表明:La掺杂量为1.5 wt%,反应液初始pH值为7,160℃水热12 h,经500℃焙烧2 h,制备的La掺杂Bi2WO6的光催化性能最佳,在模拟太阳光照射下对4-CP光催化去除率达51%,明显高于Bi2WO6的光催化活性(38.4%)。
关键词 :Bi2WO6,La掺杂,4-氯酚,光催化

Copyright © 2017 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
环境污染和能源短缺是制约人类社会发展的重要现实问题。近年来,利用半导体光催化氧化技术降解水及空气中有机污染物倍受关注 [1] 。然而,纳米TiO2带隙较宽,仅能被波长小于387 nm的紫外光激发,且光生电子-空穴的复合率较高,严重限制了其广泛应用 [2] [3] 。
Bi2WO6作为一种Aurivillius型氧化物,禁带宽度2.7 eV,比TiO2 3.2 eV的禁带宽度更窄,是一种比TiO2 可见光响应能力强的新型光催化材料 [4] [5] 。然而,Bi2WO6光催化剂的导带低电势(ECB)导致光生电子与电子受体的反应无效,增加了光生载流子复合,从而降低了光催化活性 [6] 。
将掺杂元素引入催化剂晶格形成表面缺陷,形成新的杂质能级调控禁带宽度,能够抑制光生载流子复合 [7] [8] 。镧系离子4f轨道易与各种路易斯碱(例如醇、醛、胺等)结合,广泛用于半导体光催化材料的掺杂改性剂 [9] 。Xu等 [10] 研究Gd、Eu、Sm或La掺杂的Bi2WO6在可见光下光催化降解RhB性能,La掺杂Bi2WO6样品暗吸附能力最好、光催化性能较高。
本文采用水热法制备了La掺杂Bi2WO6光催化剂,考察了掺杂量、反应液初始pH值、水热时间、焙烧温度及时间等条件因素对La掺杂Bi2WO6在模拟太阳光照射下光催化降解4-CP性能的影响,建立了水热法制备La掺杂Bi2WO6光催化剂的优化工艺。本文La掺杂量明显低于报道的La3+掺杂Bi2WO6光催化剂的最佳La3+掺杂量摩尔比为5% [11] ,水热时间明显低于Xu [10] 等报道La掺杂Bi2WO6的水热时间16 h。
2. 实验部分
2.1. 仪器和试剂
Bi(NO3)3·5H2O(天津市致远化学试剂有限公司);Na2WO4·2H2O(天津市科密欧试剂有限公司);La2O3 (天津市光复精细化工研究所);四氯酚(天津市光复精细化工研究所);浓硝酸(哈尔滨化工化学试剂厂)。
氙灯(500 W上海蓝晟电子有限公司);UV-2550紫外/可见分光光度计(日本岛津公司)。
2.2. La掺杂Bi2WO6光催化剂的制备
将Bi(NO3)3·5H2O和Na2WO4·2H2O分别按摩尔比2:1的比例称取,再称取一定量的La2O3,将4.85 g Bi(NO3)3·5H2O和La2O3溶于3.5 mL 4 M稀硝酸,1.65 g Na2WO4·2H2O溶解在5 mL 2M NaOH溶液中,将Na2WO4溶液逐滴加入Bi(NO3)3和La2O3的混合溶液中,用2 M NaOH溶液调节混合物的pH值,持续搅拌1 h后将混合物移入50 mL水热反应釜中,在160℃水热一定时间,常温下冷却。然后,将制得的淡黄色沉淀用蒸馏水洗涤3次,于110℃下在真空干燥24 h,在一定温度下焙烧得到La掺杂Bi2WO6样品。
3. 实验部分
3.1. 掺杂量对La掺杂Bi2WO6光催化活性的影响
La/Bi2WO6质量比为1 wt%、1.25 wt%、1.5 wt%、1.75 wt%、2.5 wt%、3 wt%,反应液初始pH值为7,水热12 h,再经450℃焙烧2 h制备的La掺杂Bi2WO6的光催化活性如图1所示。未掺杂的Bi2WO6光活性为38.4%,随着掺杂量增加样品光催化性能逐步升高,掺杂量为1.5 wt%的样品光活性最高,达45.8%;继续增加掺杂量,导致样品光活性降低。适量La掺杂拓宽了Bi2WO6的可见光吸收范围,增加了比表面积,抑制了光生电子-空穴的复合,并增加了传输速率,从而提高了样品的光催化活性 [12] [13] 。本文最佳La掺杂量1.5 wt%明显低于报道的La3+掺杂Bi2WO6光催化剂的最佳La3+掺杂量摩尔比为5% [11] 。
3.2. 反应液初始pH值对La掺杂Bi2WO6光催化活性的影响
反应液初始pH值对La掺杂Bi2WO6光催化活性的影响如图2所示。当反应液初始pH值为7时,制备1.5 wt% La掺杂Bi2WO6样品的光催化活性最高,达45.8%;升高或降低反应液初始pH值,制备样品的光催化活性均降低。反应液初始pH值控制溶液中各种离子的存在状态,并影响La掺杂Bi2WO6的形貌,进而影响光催化性能。强酸条件下,Na2WO4迅速水解,生成大量沉淀物H2WO4,最终生成Bi多W少的物质或可能含有少量杂质晶相Bi2O3,导致样品纯度降低 [14] [15] 。当反应液初始pH = 7时,合成的La掺杂Bi2WO6样品结晶度最好,光催化性能最好 [16] 。pH值增加到碱性条件时,OH−的浓度增大,Bi3+大量水解,Bi2WO6的成核率减小,晶核的生长速率反而提高,Bi2WO6晶核逐渐长大形成了片状结构,合成样品的平均粒径较大,导致比表面积变小,降低了光催化反应的接触面积,不利于光生电子空穴的分离,导致样品光催化活性降低 [17] 。
3.3. 水热时间对La掺杂Bi2WO6光催化活性的影响
不同水热时间制备的1.5 wt% La掺杂的Bi2WO6光催化活性如图3所示。在实验范围内,随着水热时间延长,1.5 wt% La掺杂的Bi2WO6的结晶程度逐步增大,样品光催化活性升高;当水热时间为12 h时,样品光催化活性达45.8%;继续延长水热时间,样品的光催化活性降低。究其原因,过分延长水热反应时间会导致样品晶粒尺寸增大,比表面积减小,光催化反应活性点位减少,而且晶粒尺寸增大导致光生载流子的复合几率增大,样品的光催化活性降低 [18] [19] 。本文确定的优化水热时间12 h,低于Xu [10] 等报道La掺杂Bi2WO6的水热时间16 h。
3.4. 焙烧温度对La掺杂Bi2WO6光催化活性的影响
经不同温度焙烧2 h制得的1.5 wt% La掺杂的Bi2WO6的光催化活性如图4所示。随着焙烧温度从400℃升至500℃,制备样品的光催化活性升高,经500℃焙烧样品的光催化活性最高,达51%。升高焙烧温度,样品晶化度提高,表面缺陷减少,光催化活性升高。焙烧温度继续升高导致样品光催化活性反而下降,这是由于焙烧温度过高,样品颗粒过大,比表面积降低所致。
3.5. 焙烧时间对La掺杂Bi2WO6光催化活性的影响
经500℃焙烧不同时间制备的1.5 wt% La掺杂的Bi2WO6的光催化活性如图5所示。结果表明,经500℃焙烧2 h制备的样品催化活性最佳,延长或缩短焙烧时间均导致样品光催化活性降低。焙烧时间过

Figure 1. Effect of La-doping amount on the photoactivity of La-doped Bi2WO6
图1. 掺杂量对La掺杂Bi2WO6光催化活性的影响
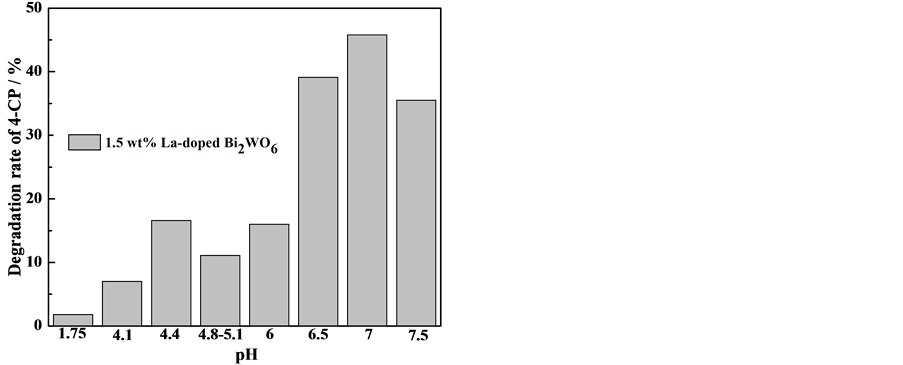
Figure 2. Effect of the initial pH of reactant solution on the photoactivity of La-doped Bi2WO6
图2. 反应液初始pH值对La掺杂Bi2WO6光催化活性的影响

Figure 3. Effect of hydrothermal time on the photoactivity of La-doped Bi2WO6
图3. 水热时间对La掺杂Bi2WO6光催化活性的影响

Figure 4. Effect of calcination temperature on the photoactivity of La-doped Bi2WO6
图4. 焙烧温度对La掺杂Bi2WO6光催化活性的影响

Figure 5. Effect of calcination time on the photoactivity of La-doped Bi2WO6
图5. 焙烧时间对La掺杂Bi2WO6光催化活性的影响
短,样品表面缺陷过多,晶化度较低,样品光催化活性较低;焙烧时间过长,晶粒过大,比表面降低,量子化效率变低,也会导致样品的光催化活性降低。
4. 结论
La掺杂明显提高了Bi2WO6的光催化活性。经实验研究,确定水热法制备La掺杂Bi2WO6的优化工艺条件如下:La掺杂量为1.5 wt%,反应液初始pH值为7,160℃下水热时间为12 h,焙烧温度为500℃,焙烧时间2 h,在此条件下制备的La掺杂Bi2WO6在模拟太阳光照射下光催化降解4-CP的去除率达到51%,远高于相同条件下制备的Bi2WO6的光催化活性(38.4%)。与文献报道相比,La掺杂量明显降低,水热时间显著缩短,该工艺具备降耗节能的特点。
基金项目
黑龙江省自然科学基金(No.E201323);黑龙江省教育厅科学技术研究项目(No.12531213)。
文章引用
沈俊玲,姜洪泉. La掺杂Bi2WO6光催化剂的水热制备工艺
Hydrothermal Preparation of La-Doped Bi2WO6 Photocatalyst[J]. 材料科学, 2017, 07(03): 254-259. http://dx.doi.org/10.12677/MS.2017.73035
参考文献 (References)
- 1. Xu, J.H., Wang, W.Z., Sun, S.M. and Wang, L. (2012) Enhancing Visible-Light-Induce Photocatalytic Activity by Coupling with Wide-Band-Gap Semiconductor. A Case Study on Bi2WO6/TiO2. Applied Catalysis B: Environmental, 111, 126-132. https://doi.org/10.1016/j.apcatb.2011.09.025
- 2. Fenoll, J., Martinez-Menchon, M., Navarro, G., Vela, N. and Navarro, S. (2013) Photocatalytic Degradation of Substiuted Phenylurea Herbicides in Aqueous Semi-conductor Suspensions Exposed to Solar Energy. Chemosphere, 91, 571-578. https://doi.org/10.1016/j.chemosphere.2012.11.067
- 3. 姜洪泉, 李振宇, 王雪峰, 李井申, 王巧凤. TiCl4水解法水热制备Yb-P-TiO2纳米光催化剂及共掺杂的协同机制[J]. 化工学报, 2014, 65(12): 5030-5038.
- 4. Ricote, J., Pardo, L., Castro, A. and Millán, P. (2001) Study of the Process of Mechano-chemical Activation to Obtain Aurivillius Oxides with n = 1. Journal of Solid State Chemistry, 160, 54-61. https://doi.org/10.1006/jssc.2001.9188
- 5. Tian, Y.L., Chang, B.B., Lu, J.L., Fu, J., Xi, F.N. and Dong, X.Q. (2013) Hydrothermal Synthesis of Graphitic Carbon Ni-tride-Bi2WO6 Heterojunctions with Enhanced Visible Light Photocatalytic Activities. ACS Applied Materials & Interfaces, 5, 7079-7085. https://doi.org/10.1021/am4013819
- 6. Sun, S.M., Wang, W.Z. and Zhang, L. (2012) Efficient Contaminant Removal by Bi2WO6 Films with Nanoleaflike Structures through a Photoelectrocatalytic Process. The Journal of Physical Chemistry C, 116, 19413-19418. https://doi.org/10.1021/jp306332x
- 7. Zhang, L.S., Wang, H.L., Chen, Z.G., Wong, P.K. and Liu, J.S. (2011) Bi2WO6 Micro/nano-Structures: Synthesis, Modifications and Visible-Light-Driven Photocatalytic Applications. Applied Catalysis B: Environmental, 106, 1-13. https://doi.org/10.1016/j.apcatb.2011.05.008
- 8. Bhattacharya, C., Lee, H.C. and Bard, A.J. (2013) Rapid Screening by Scanning Electrochemical Microscopy (SECM) of Dopants for Bi2WO6 Improved Photocatalytic Water Oxidation with Zn Doping. The Journal of Physical Chemistry C, 117, 9633-9640. https://doi.org/10.1021/jp308629q
- 9. Ranjit, K.T., Willner, I., Bossmann, S.H. and Braun, A.M. (2001) Lan-thanide Oxide-Doped Titanium Dioxide Photocatalysts: Novel Photocatalysts for the Enhanced Degradation of p-Chlorophenoxyacetic Acid. Environmental Science & Technology, 35, 1544-1549. https://doi.org/10.1021/es001613e
- 10. Xu, X.T., Ge, Y.X., Li, B., Fan, F.L. and Wang, F. (2014) Shape Evolution of Eu-Doped Bi2WO6 and Their Photocatalytic Properties. Materials Research Bulletin, 59, 329-336. https://doi.org/10.1016/j.materresbull.2014.07.050
- 11. 王春英, 谷传涛, 朱清江, 阴梦如, 罗仙平, 余长林. 稀土La3+掺杂Bi2WO6光催化降解活性艳红X-3B的研究[J].中国环境科学, 2015, 35(7): 2007-2013.
- 12. Zhang, W.H., Yu, N., Zhang, L.S., Jiang, K.W., Chen, Y.Z. and Chen, Z.G. (2016) Synthesis of Yb3+/Er3+ Co-Doped Bi2WO6 Nanosheets with Enhanced Photocatalytic Activity. Materials Letters, 163, 16-19. https://doi.org/10.1016/j.matlet.2015.09.113
- 13. Zhang, C. and Zhu, Y.F. (2005) Synthes is of Square Bi2WO6 Nanoplates as High Activity Visible Light Driven Photocatalysts. Chemistry of Materials, 17, 3537-3545. https://doi.org/10.1021/cm0501517
- 14. He, Z., Sun, C., Yang, S.G., Ding, Y.C., He, H. and Wang, Z.L. (2009) Photocatalytic Degradation of Rhodamine B by Bi2WO6 with Electron Accepting Agent under Microwave Irradiation: Mechanism and Pathway. Journal of Hazardous Materials, 162, 1477-1486. https://doi.org/10.1016/j.jhazmat.2008.06.047
- 15. 卓艺乔, 黄剑锋, 曹丽云, 吴建鹏. 钨与铋摩尔比对Bi2WO6微晶形貌及光学性能的影响[J]. 硅酸盐学报, 2012, 40(6): 913-921.
- 16. 刘瑛, 王为民, 傅正义, 王皓, 王玉成, 张金咏. Bi2WO6的水热合成及其光催化性能研究[J]. 无机材料学报, 2011, 26(11): 1169-1174.
- 17. Sun, Q., Jia, X.R., Wang, X.F., Yu, H.G. and Yu, J.G. (2015) Facile Synthesis of Porous Bi2WO6 Nanosheets with High Photocatalytic Performance. Dalton Transactions, 44, 14532-14539. https://doi.org/10.1039/C5DT01859E
- 18. Shang, M., Wang, W.Z., Sun, S.M., Zhou, L. and Zhang, L. (2008) Bi2WO6 Nanocrystals with High Photocatalytic Activities under Visible Light. The Journal of Physical Chemistry C, 112, 10407-10411. https://doi.org/10.1021/jp802115w
- 19. Zhang, C. and Zhu, Y.F. (2005) Synthesis of Square Bi2WO6 Nanoplates as High-Activity Visible-Light-Driven Photocatalysts. Chemistry of Materials, 17, 3537-3545. https://doi.org/10.1021/cm0501517