International Journal of Psychiatry and Neurology
Vol.06 No.03(2017), Article ID:21371,9
pages
10.12677/IJPN.2017.63004
Cerebral Microbleeds Screening with Original Image of 3D-TOF MRA Sequences Predicting Hemorrhagic Transformation in the Patients with Cerebral Infarction
Yongpeng Yu1*, Lijun Liu2
1Department of Neurology and Central Laboratory of Brain Diseases, The Affiliated Weihai Central Hospital of Qingdao University, Weihai Shandong
2Department of Neurology, The Affiliated Hospital of Qingdao University, Qingdao Shandong

Received: Jun. 26th, 2017; accepted: Jul. 10th, 2017; published: Jul. 14th, 2017

ABSTRACT
Objective: To investigate the feasibility of the original image of the three-dimensional time of flight MR angiography (Three dimensional time-of-flight magnetic resonance angiography, 3D- TOF MRA) assessing cerebral microbleeds (CMBs) in the patients with cerebral infarction, and further investigate the application value of 3D-TOF sequence in predicting hemorrhagic transformation from cerebral infarction. Methods: 192 cases of patients with cerebral infarction were enrolled into this study, and susceptibility weighted imaging (SWI) and 3D-TOF sequence scanning were performed to assess the ability of SWI and original axial images of 3D-TOF MRA showing CMBs. We investigated the imaging signal distribution of hemorrhagic transformation with cerebral infarction in the original image of CT, T1WI, DWI, SWI and 3D-TOF MRA sequences and the characteristics of CMBs to clarify the relationship between the number grade of lesions and hemorrhagic transformation a clear sequence of CMBs based on 3D-TOF sequence, and explored the application value of 3D-TOF predicting hemorrhagic transformation with cerebral infarction. Results: 12 cases were excluded for the unclear imaging data. SWI found 264 lesions of CMBs among 180 cases with cerebral infarction. Classification of CMBs: 28 cases of mild, 8 cases of moderate, and 4 cases of severe. The original sequences of 3D-TOF MRA found 188 lesions of CMBs including 24 cases of mild, four cases of moderate, and four cases of severe. There was statistically significant difference in the CMBs lesions detection rate between these two sequences (cortical vs. subcortical, thalamus, cerebellum, p < 0.05). There was no significant difference in CMBs lesions detection rate in the basal ganglia and brainstem between them (p < 0.05). Among 180 patients, there were a total of 24 cases of hemorrhagic transformation (13.3%). The detection sensitivities of hemorrhagic transformation of T1WI, DWI, SWI and 3D-TOF MRA sequence were 25.0%, 62.5%, 100% and 83.3%. CMBs lesions number was positively correlated with the incidence of hemorrhagic transformation. Conclusion: In the absence of SWI sequence, the original image of 3D-TOF MRA sequence can effectively display CMBs lesions, which can be used as an effective means of predicting hemorrhagic transformation of cerebral infarction.
Keywords:Cerebral Infarction, Cerebral Microbleeds, Hemorrhagic Transformation, 3D-TOF MRA, SWI
3D-TOF MRA序列筛查脑微出血 在脑梗死出血性转化中的预警价值
于永鹏1*,刘丽君2
1青岛大学附属威海市中心医院神经内科,中心实验室脑病研究室,山东 威海
2青岛大学附属医院黄岛分院神经内科,山东 青岛

收稿日期:2017年6月26日;录用日期:2017年7月10日;发布日期:2017年7月14日

摘 要
目的:探讨三维时间飞跃法磁共振血管成像(Three dimensional time-of-flight magnetic resonance angiography, 3D-TOF MRA)原始图像评估脑梗死患者脑微出血(Cerebral microbleeds, CMBs)病灶的可行性,进一步探讨3D-TOF序列在预测脑梗死出血性转化中的应用价值。方法:选取192例脑梗死患者为研究对象,分别进行磁敏感加权成像(Susceptibility weighted imaging, SWI)和3D-TOF序列扫描,评估SWI及3D-TOF MRA原始轴位图像显示CMBs的优劣。观察脑梗死出血性转化在CT、T1WI、DWI、SWI及3D-TOF MRA序列原始图像中的影像学表现及CMBs的分布和信号特点,明确基于3D-TOF序列CMBs病灶数量分级与出血性转化的关系,探讨3D-TOF序列在预测脑梗死出血性转化中的应用价值。结果: 因影像学资料显示不清排除12例,实际纳入180例脑梗死患者,SWI共发现微出血病灶264个,CMBs数目分级:28例为轻度,8例为中度,4例为重度。3D-TOF MRA序列原始图像发现微出血病灶188个,其中24例为轻度,4例为中度,4例为重度。两种序列对微出血病灶的检出率比较差异有统计学意义(皮质和皮质下、丘脑、小脑,p < 0.05);两者对基底节和脑干的微出血病灶检出率差异无统计学意义(p < 0.05)。180例患者中,24例发生出血性转化(13.3%),T1WI、DWI、SWI及3D-TOF MRA序列检测出血性转化的敏感度分别为25.0%、62.5%、100%及83.3%。CMBs病灶数量与出血性转化的发生率呈正相关。结论:在缺乏SWI序列的情况下,3D-TOF MRA原始图像可有效显示CMBs病灶,可作为预测脑梗死发生出血性转化的有效手段之一。
关键词 :脑梗死,脑微出血,出血性转化,三维时间飞跃法磁共振血管成像,磁敏感加权成像

Copyright © 2017 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
脑微出血(Cerebral microbleeds, CMBs)是脑小血管病变所致的、以微小出血为主要特征的一种脑实质
亚临床损害 [1] ,属于脑小血管病(Cerebral small vessel disease, CSVD)的范畴。作为一种有出血倾向的微小血管病变标志,脑微出血(Cerebral microbleeds, CMBs)在各种脑血管病中的发生及其意义正日益受到国内外研究者和临床医生的重视,成为目前脑血管病研究领域的热点问题。脑梗死的出血性转化是指脑梗死继发脑出血,发生率为8.5%~30%,随着CT和MRI应用的普及以及抗凝与溶栓药物的广泛应用,出血性转化的报道有逐渐增多的趋势 [2] 。研究提示CMBs与脑梗死的出血转化和自发性脑出血复发的风险高度相关 [3] 。CMBs亦能增加溶栓或抗血小板治疗后出血的风险 [4] ,因此出血性转化增加了缺血性脑卒中的临床决策难度及其不良预后。鉴于T2加权梯度回波(T2*GRE)及磁敏感加权成像(Susceptibility weighted imaging, SWI)序列扫描时间较长,目前大部分医院并未将T2*GRE及SWI序列列为常规检查,如能开发和利用现有常规扫描序列的潜在用途,最大限度利用现有其他影像技术检测CMBs是个具有临床应用价值的研究方向,有利于提高成本–效价比及设备的使用效率。目前三维时间飞跃法磁共振血管成像(Three dimensional time-of-flight magnetic resonance angiography, 3D-TOF MRA)已经成为脑梗死临床实践中血管评估的常规手段,本研究旨在探讨采用3D-TOF MRA原始图像评估脑梗死合并CMBs的可行性,进一步探讨3D-TOF MRA原始图像在预测脑梗死出血性转化中的应用价值,为评估CMBs及预测脑梗死发生出血性转化提供经济而有效的手段,为最大限度发挥常规影像学检测技术的应用范围提供依据。
2. 材料与方法
2.1. 研究对象
收集就诊于青岛大学附属威海市中心医院神经内科192例急性缺血性脑卒中患者,其中男116例,女76例,年龄50~89岁,平均68.2岁。纳入标准:1) 所有病例诊断符合中国急性缺血性脑卒中诊治指南2010版的标准;2) 发病48小时以内的脑梗死患者;3) 首次发病或既往有卒中史,但未遗留明显后遗症;4) 除外腔隙性脑梗死;5) 首次CT或MRI检查除外脑出血(不包括SWI检出的微出血)。排除标准:1) 合并颅内出血、感染及占位性等病变;2) 存在出血素质、严重凝血功能及肝、肾功能异常;3) 存在磁共振检查禁忌证(例如体内存在非顺磁物质或心脏起搏器等);本研究项目符合当地医疗机构伦理委员会伦理标准并获得所有患者或其家属的知情同意。
2.2. 影像学检测方法
采用GE公司的超导性磁共振成像系统(型号:GE MR Discovery 750 3.0T)、先后获得横轴位T1WI及T2WI、矢状位T1WI、DWI、3D-TOF MRA和SWI图像。扫描参数为:FLAIR-T1WI(TR/TE,1750 ms/24 ms,TI 780 ms;FOV 24 cm × 24 cm,矩阵320 × 256);FRFSE-T2WI (TR/TE,4300 ms/95 ms,FOV 24 cm × 24 cm,矩阵512 × 512);DWI-EPI (TR/TE,3000 ms/70 ms;FOV24 cm × 24 cm,矩阵160×160)。其中SWI (SWAN)序列参数为:3D T1-FFE,TR Minimum,TE 45.0 ms,反转角15˚,层厚2 mm,无间隔采集,矩阵384 × 320,激励次数1.0。选择入组的患者在首次MRI检查后1至4周内病情变化或复诊时复查MRI平扫 + 3D-TOF MRA (加扫SWI)。
2.3. 图像处理及资料分析
所有MR图像均由2名有经验的神经放射学医师采用盲法共同分析,根据CT、T1WI、SWI的动态变化明确是否发生出血性转化,并记录其在各序列的信号特征。CMBs定义为SWI序列上卵圆形或环形的均匀低信号区,直径为2~5 mm,周围无水肿,于常规序列上未能显示,并除外小静脉及钙化灶(图1A)。3D-TOF序列原始图像上,微出血病灶表现为脑实质内点状或圆形低信号影,其信号较脑脊液信号更低,
周围可伴轻度伪影(图1B)。梗死区小静脉异常显示表现为SWI序列上粗细不等、细线状低信号,则确定为小静脉影,予以排除。DWI表现为类圆形低信号影,DWI显示病灶的边界不及SWI序列清晰,且病灶显示相对较小(图1C)。出血性转化确定标准:发病后首次检查(包括CT和MRI)未发现脑内急性出血,复查MRI发现脑内不同范围梗死病灶内的短T1信号病灶,或等T1、短T2信号灶、SWI序列及3D-TOF序列低信号灶(对照CT除外钙化灶)。参照既往研究 [5] ,CMBs按照数目分级:无CMBs为0;轻度,CMBs为1~4个;中度:CMBs为5~9个,重度:CMBs ≥ 10个。由于3D-TOF MRA轴位原始图像仅扫描范围至胼胝体上缘,因此,本研究仅评估在颅底至胼胝体上缘范围内脑实质微出血情况。
2.4. 统计学分析
采用SPSS 17.0统计软件,正态分布定量资料结果以均数 ± 标准差表示,两样本均数比较采用t检验,计数资料两样本间比较采用卡方检验,由于CMBs分布不同,采用Friedman检验不同序列检测CMBs能力差异性进行检验,p < 0.05表示差异有统计学意义。
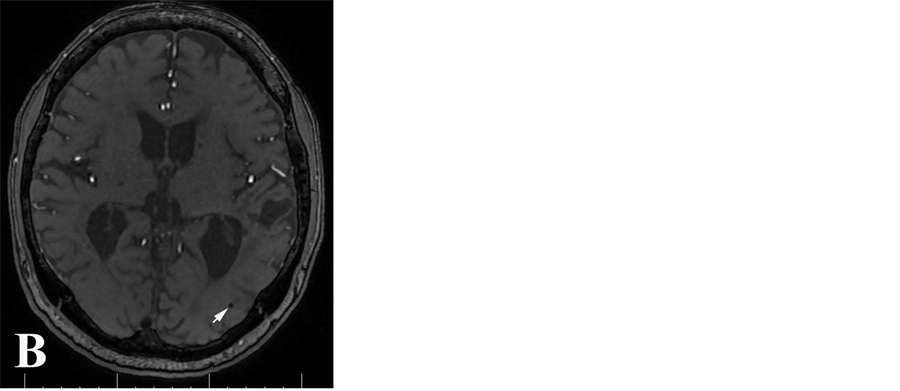
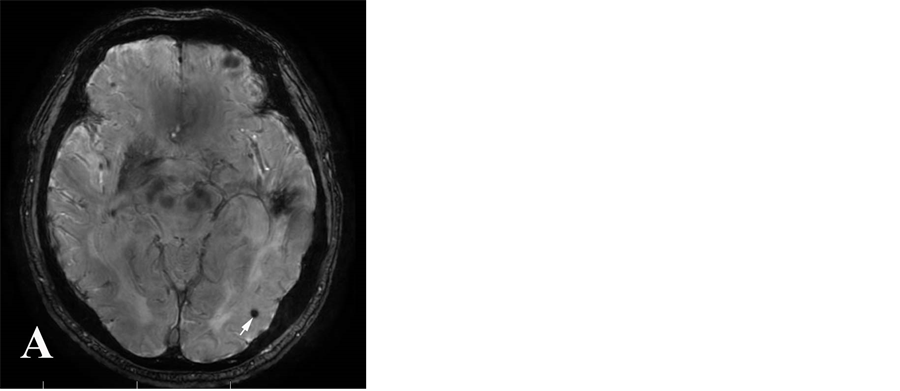
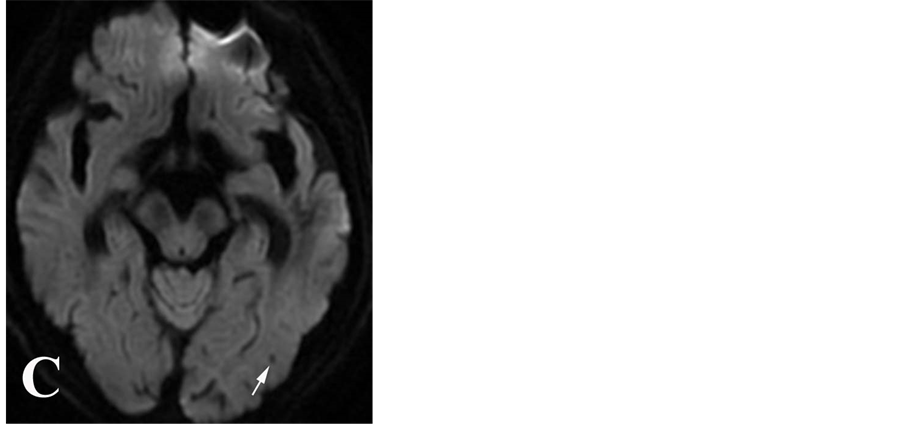
Figure 1. Cerebral microbleeds in SWI image (A), original image of 3D-TOF MRA sequences (B) and DWI image (C)
图1. 脑微出血在SWI像(A)、3D-TOFMRA 原始图像(B)及DWI像(C)的表现
3. 结果
3.1. 脑微出血检测结果
192例脑梗死患者中,初次SWI序列显示有52例脑实质存在CMBs,占总病例数的27.1%。排除12例8例SWI序列显示CMBs位于胼胝体上缘以上,4例因TOF序列原始图像质量差而无法评估。其余40例中,CMBs数目分级:7例为轻度,2例为中度,1例为重度。共发现微出血病灶260个。40例脑梗死患者中,3D-TOF MRA序列原始图像显示32例存在微出血病灶,其中24例为轻度,4例为中度,4例为重度,共发现病灶188个。
3.2. SWI和3D-TOF MRA序列原始图对不同部位CMBs病灶检出率的比较
SWI序列可以显示皮质、皮质下、基底节、丘脑、小脑及脑干等部位的微出血病灶。而3D-TOF MRA序列原始图像显示丘脑和基底节区病灶更为多见。T检验显示,两种序列显示丘脑、小脑及皮质区病灶的能力差异有统计学意义(p < 0.01)。而对发现基底节区及脑干微出血病灶的能力差异无统计学意义(p > 0.05)。总的来看,SWI序列图像显示CMBs病灶的总数量大于3D-TOF MRA序列原始图像。以SWI序列为评价标准,3D-TOF MRA序列原始图像对CMBs病灶的检出率为72.3%。对皮质及皮质下、脑干、小脑、丘脑的检出率分别为52.6%、85.7%、60.0%、66.7%。3D-TOF MRA序列原始图像对于发现基底节区微出血病灶能力与SWI序列相当。8例脑梗死患者在SWI序列检出微出血灶,而在3D-TOF MRA序列原始图像未予显示(见表1)。
3.3. 3.0T MR多序列成像对出血性转化检出率的比较
180例患者中共24例经随访证实发生出血性转化,出血性转化发生率为13.3%。T1WI、DWI、SWI及3D-TOF序列发现出血性转化的敏感度分别为25.0%、62.5%、100%及83.3% (见表2)。SWI及3D-TOF序列对出血性转化的检出优于DWI (p < 0.05)。
3.4. 基于3D-TOF MRA序列原始图像的CMBs病灶数量分级与出血性转化的关系
180例患者中,无CMBs 148例,其中10例发生出血性转化;轻度CMBs 24例,6例发生出血性转化;中–重度CMBs 8例,皆发生出血性转化。随CMBs程度加重,出血性转化发生的风险明显增加(见表3)。
4. 讨论
CMBs已成为影响脑梗死后出血转化、预后及复发、造成认知障碍以及年龄相关能力丧失的重要危险因素 [6] [7] [8] [9] ,目前尚无有效的防治手段。由于该病呈缓慢进展,一般缺乏相应的临床症状和体征,且常规头颅CT和MRI检测对其不敏感,故以前很少被发现和重视。由于新的MRI技术的推广与应用,尤其是T2*GRE序列及SWI,越来越多的CMBs患者被确诊。
4.1. SWI与3D-TOF序列成像原理比较
SWI是一项新近发展起来的磁共振成像技术,GE公司称SWI为ESWAN,它是利用血氧水平依赖效应(如血液或铁)和局部不同组织间磁敏感性的差异而成像的技术。它是一个三维采集、完全流动补偿的、高分辨率的,以T2*WI加权梯度回波序列为基础,经薄层重建得到的强度图像和相位图像。这种序列增加组织间的磁敏感差异,使敏感性最大化,其独特的数据采集和图像处理过程提高了图像的对比,最小密度图使高信号脑组织信号最小化,对静脉血及细小静脉、微出血和铁沉积及钙化高度敏感。近年来,研究认为SWI序列是诊断缺血性脑卒中出血性转化的最敏感序列之一 [10] [11] ,6 h内对出血灶检出的敏
Table 1. Comparison in detection of different parts of microbleeds used by SWI and original image of 3D-TOF MRA sequences
表1. SWI和3D-TOF MRA序列原始图对不同部位微出血病灶检出情况比较
SWI:磁敏感加权成像;3D-TOF:三维时间飞跃法磁共振血管成像。
Table 2. Comparison of detection rate of hemorrhagic transformation used by 3.0T MR multi-sequence imaging
表2. 3.0T MR多序列成像对出血性转化检出率的比较
Table 3. The relationship between the number of CMBs lesions and the degree of hemorrhagic transformation based on the original image of 3D-TOFMRA sequence
表3. 基于3D-TOFMRA序列原始图像CMBs病灶数量分级与出血性转化的关系
感性和特异性均可达到100%。但是临床上加扫SWI序列耗时较长,增加了设备使用的成本和医疗费用。3D-TOF序列同样是一个梯度回波序列,同样具有磁敏感效应,能够显示引起磁场不均匀的因素如微出血或钙化等。3D-TOF序列在临床实践中使用广泛,3D-TOF序列原始图像上,微出血病灶表现为脑实质内点状或圆形低信号影,其信号较脑脊液信号更低,周围可伴轻度伪影。该序列的磁敏感效应虽不及T2*GRE及SWI序列明显,但该序列在临床实践中应用广泛,比较容易获取。因此,探讨该序列对CMBs评估的应用价值是非常有必要的。
4.2. 3D-TOF序列在缺血性卒中患者CMBs评估中的价值
目前已被证实CMBs病灶与多种血管性病变存在密切关系,因此CMBs评估和检测在临床实践中越来越受到关注。研究显示,CMBs灶在出血性或缺血性脑血管病患者及健康人群中皆可发生。由于研究人群的选择性差异,CMBs发生率在不同研究中有所不同 [12] [13] 。本研究约有26.7%的入组病例存在CMBs,与既往研究的结果基本吻合 [14] [15] 。本研究设定SWI检出率为100%作为对比,3D-TOF MRA原始图像能够检出大部分微出血病灶,检出率约72%。虽然3D-TOF MRA原始图像对CMBs总体检出率不及SWI序列,但对发现基底节区微出血灶的敏感性稍高于SWI序列。鉴于CMBs在临床评估中的重要意义,3D-TOF MRA原始图像可有效显示CMBs病灶,特别是在缺乏SWI或T2*GRE序列图像的情况下,对3D-TOF MRA原始图像的评估可为脑卒中患者治疗方法的选择和预后判断提供依据,有利于最大限度的发挥常规扫描序列的潜在用途,提高影像学检测技术临床应用的成本–效益比。
4.3. 3D-TOF序列对脑梗死出血性转化的预测价值
4.3.1. CMBs与脑梗死出血性转化的关系
CMBs又称腔隙性出血或瘀点性出血,是微小血管病变导致微量血液外渗。对于合并CMBs急性脑梗死患者,溶栓是否会增加出血性转化的发生风险,至今国内外研究还没有统一的定论。新近研究表明随着脑梗死患者CMBs负荷的增加,脑出血风险轻度增加 [12] [16] 。最近一项基于我国人群的研究发现,CMBs的存在与出血性转化风险无关 [17] 。另外也有研究提示现有的证据并不能证明合并CMBs脑梗死患者溶栓治疗能显著增加出血风险 [18] 。脑出血的存在,特别是多发脑微出血使死亡风险增加 [19] 。本研究发现合并CMBs的急性脑梗死患者在随访过程中出血性转化的发生率达到62.5%。发现CMBs的数目与发生出血性转化的风险有相关性 [20] ,本研究中2例中–重度CMBs患者皆发生了出血性转化,发生率明显高于轻度及无CMBs患者。本研究与既往结果有出入,考虑与检测微出血的手段有关系,如果检测CMBs的手段敏感性高,则对预测出血转化的特异性就降低,该研究所采用3D-TOF MRA原始图像对CMBs检测的敏感性较低,因此,一旦发现存在脑梗死合并CMBs,对其出血转化的预警价值较高。因此,一般认为对于存在多发CMBs患者溶栓及抗凝治疗应警惕出血转的风险,特别是先前有多发脑梗死病史或曾经有脑出血病史的患者,如果能够在入院后及早行MRI平扫 + 3D-TOF MRA检查,一方面可以评估患者颅内血管病变情况,另一方面可以在原始图像上评估是否存在广泛的CMBs,对制定下一步的治疗决策有重要的参考价值。
4.3.2. 基于3D-TOF序列的CMBs病灶数量分级可有效预测脑梗死出血性转化
既往诸多研究探讨基因多态与微出血 [21] [22] 及出血转化 [23] [24] [25] [26] 的关系。也有研究探讨血清炎性因子与微出血及小血管疾病之间的联系 [27] 。通过基因多态性或炎性因子检测来预测脑出血转化,对于临床实践中的急性脑梗死的处置的时间紧迫性来讲,上述检测较耗时费力,且影响因素较多,直观性不佳。因此本研究着眼于磁共振常规序列扫描的潜在应用价值,探讨基于3D-TOF序列的CMBs病灶评估预测脑梗死出血性转化的发生风险,研究证实,3D-TOF序列对CMBs病灶检出的敏感性不及SWI序列,但对出血转化的预测特异性较高,对缺血性卒中临床处置有较高的指导价值,凸显了该研究对改善影像学检测的成本–效益比及提高设备使用效率的贡献。然而本研究纳入的样本较少,研究结果需要将来大样本前瞻研究加以证实。
5. 小结
综上所述,鉴于CMBs评估在脑梗死临床实践中的重大意义,作为缺血性脑卒中患者的临床常规检查手段,3D-TOF MRA原始图像能敏感显示胼胝体上缘以下特别是基底节及脑干的CMBs病灶,可有效预测脑梗死出血性转化的发生。尤其是在缺乏SWI或T2*GRE序列图像的情况下,该序列的原始图像可为脑梗死患者治疗方法的选择和预后判断提供依据,有助于最大限度发挥常规扫描序列的潜在用途,提高影像学检测技术临床应用的成本–效益比和设备使用效率。
基金项目
本研究得到以下基金项目资助:国家自然科学基金项目(81400957)。
文章引用
于永鹏,刘丽君. 3D-TOF MRA序列筛查脑微出血在脑梗死出血性转化中的预警价值
Cerebral Microbleeds Screening with Original Image of 3D-TOF MRA Sequences Predicting Hemorrhagic Transformation in the Patients with Cerebral Infarction[J]. 国际神经精神科学杂志, 2017, 06(03): 18-26. http://dx.doi.org/10.12677/IJPN.2017.63004
参考文献 (References)
- 1. 冯洁, 刘运海. 脑微出血研究进展[J]. 中国神经免疫学和神经病学杂志, 2012, 19(3): 157-160.
- 2. Lindley, R.I., Wardlaw, J.M., Sandercock, P.A., et al. (2004) Frequency and Risk Factors for Spontaneous Hemorrhagic Transformation of Cerebral Infarction. Journal of Stroke and Cerebrovascular Diseases, 13, 235-246.
- 3. Nighoghossian, N., Hermier, M., Adeleine, P., et al. (2002) Old Microbleeds Are a Potential Risk Factor for Cerebral Bleeding after Ischemic Stroke: A Gradient-Echo T2*-Weighted Brain MRI Study. Stroke, 33, 735-742. https://doi.org/10.1161/hs0302.104615
- 4. Derex, L., Nighoghossian, N., Hermier, M., et al. (2004) Thrombolysis for Ischemic Stroke in Patients with Old Microbleeds on Pretreatment MRI. Cerebrovascular Diseases, 17, 238-241. https://doi.org/10.1159/000076123
- 5. Lee, S.H., Bae, H.J., Yoon, B.W., et al. (2002) Low Concentration of Serum Total Cholesterol Is Associated with Multifocal Signal Loss Lesions on Gradient-Echo Magnetic Resonance Imaging: Analysis of Risk Factors for Multifocal Signal Loss Lesions. Stroke, 33, 2845-2849. https://doi.org/10.1161/01.STR.0000036092.23649.2E
- 6. Lee, S., Bae, H., Kwon, S., et al. (2004) Cerebralm Icrobleeds Are Regionally Associated with Intracerebral Hemorrhage. Neurology, 62, 72-76. https://doi.org/10.1212/01.WNL.0000101463.50798.0D
- 7. Roob, G., Lechner, A., Schmidt, R., et al. (2000) Frequency and Location of Microbleeds in Patients with Primary Intracerebral Hemorrhage. Stroke, 31, 2665-2669. https://doi.org/10.1161/01.STR.31.11.2665
- 8. Ueno, H., Naka, H., Ohshita, T., et al. (2008) Association between Cerebral Microbleeds on T2 Weighted MR Images and Recurrent Hemorrhagic Stroke in Patients Treated with Warfarin Following Ischemic Stroke. American Journal of Neuroradiology, 29, 1483-1486. https://doi.org/10.3174/ajnr.A1120
- 9. Werring, D.J., Frazer, D.W., Coward, L.J., et al. (2004) Cognitive Dysfunction in Patients with Cerebral Microbleeds on T2 Weighted Gradient-Echo MRI. Brain, 127, 2265-2275. https://doi.org/10.1093/brain/awh253
- 10. Schrag, M. and Greer, D.M. (2014) Clinical Associations of Cerebral Microbleeds on Magnetic Resonance Neuroimaging. Journal of Stroke and Cerebrovascular Diseases, 23, 2489-2497.
- 11. Wang, X., Wei, X.E., Li, M.H., et al. (2014) Microbleeds on Susceptibility-Weighted MRI in Depressive and Non- Depressive Patients after Mild Traumatic Brain Injury. Neurological Sciences, 35, 1533-1539. https://doi.org/10.1007/s10072-014-1788-3
- 12. Gratz, P.P., El-Koussy, M., Hsieh, K., et al. (2014) Preexisting Cerebral Microbleeds on Susceptibility-Weighted Magnetic Resonance Imaging and Post-Thrombolysis Bleeding Risk in 392 Patients. Stroke, 45, 1684-1688. https://doi.org/10.1161/STROKEAHA.114.004796
- 13. Patel, B., Lawrence, A.J., Chung, A.W., et al. (2013) Cerebral Microbleeds and Cognition in Patients with Symptomatic Small Vessel Disease. Stroke, 44, 356-361. https://doi.org/10.1161/STROKEAHA.112.670216
- 14. Vernooij, M.W., van der Lugt, A., Ikram, M.A., et al. (2008) Prevalence and Risk Factors of Cerebral Microbleeds: The Rotterdam Scan Study. Neurology, 70, 1208-1214. https://doi.org/10.1212/01.wnl.0000307750.41970.d9
- 15. Koennecke, H.C. (2006) Cerebral Microbleeds on MRI: Prevalence, Associations, and Potential Clinical Implications. Neurology, 66, 165-171. https://doi.org/10.1212/01.wnl.0000194266.55694.1e
- 16. Dannenberg, S., Scheitz, J.F., Rozanski, M., et al. (2014) Number of Cerebral Microbleeds and Risk of Intracerebral Hemorrhage after Intravenous Thrombolysis. Stroke, 45, 2900-2905. https://doi.org/10.1161/STROKEAHA.114.006448
- 17. Wang, B.G., Yang, N., Lin, M. and Lu, B. (2014) Analysis of Risk Factors of Hemorrhagic Transformation after Acute Ischemic Stroke: Cerebral Microbleeds Do Not Correlate with Hemorrhagic Transformation. Cell Biochemistry and Biophysics, 70, 135-142. https://doi.org/10.1007/s12013-014-9869-8
- 18. Charidimou, A., Kakar, P., Fox, Z., et al. (2013) Cerebral Microbleeds and the Risk of Intracerebral Haemorrhage after Thrombolysis for Acute Ischaemic Stroke: Systematic Review and Meta-Analysis. Journal of Neurology, Neurosurgery, and Psychiatry, 84, 277-280. https://doi.org/10.1136/jnnp-2012-303379
- 19. Akoudad, S., Ikram, M.A., Koudstaal, P.J., et al. (2013) Cerebral Microbleeds and the Risk of Mortality in the General Population. European Journal of Epidemiology, 28, 815-821. https://doi.org/10.1007/s10654-013-9854-3
- 20. Hermier, M., Nighoghossian, N., Derex, L., et al. (2003) Hypointense Transcerebral Veins at T2*-Weighted MRI: A Marker of Hemorrhagic Transformation Risk in Patients Treated with Intravenous Tissue Plasminogen Activator. Journal of Cerebral Blood Flow & Metabolism, 23, 1362-1370. https://doi.org/10.1097/01.WCB.0000091764.61714.79
- 21. Loehrer, E., Ikram, M.A., Akoudad, S., et al. (2014) Apolipo-protein E Genotype Influences Spatial Distribution of Cerebral Microbleeds. Neurobiology of Aging, 35, 899-905.
- 22. Lanfranconi, S., Franco, G., Borellini, L., et al. (2013) Genetics of Cerebral Hemorrhage and Microbleeds. Panminerva Medica, 55, 11-28.
- 23. Fernandez-Cadenas, I., Alvarez Sabin, J., Molina, C.A., et al. (2006) ApoE Genotype Influences on Efficacy and Safety of Thrombolytic Treatment for Ischemic Stroke. Neurologia, 21, 176-180.
- 24. Zhang, X., Cao, X., Xu, X., et al. (2015) Correlation between the -1562C/T Polymorphism in the Matrix Metalloproteinase-9 Gene and Hemorrhagic Transformation of Ischemic Stroke. Experimental and Therapeutic Medicine, 9, 1043-1047. https://doi.org/10.3892/etm.2015.2186
- 25. Mallolas, J., Rodríguez, R., Gubern, C., et al. (2014) A Polymorphism in the Promoter Region of the Survivin Gene Is Related to Hemorrhagic Transformation in Patients with Acute Ischemic Stroke. NeuroMolecular Medicine, 16, 856- 861. https://doi.org/10.1007/s12017-014-8333-7
- 26. Appelboom, G., Piazza, M., Bruce, S.S., et al. (2012) Variation in a Locus Linked to Platelet Aggregation Phenotype Predicts Intraparenchymal Hemorrhagic Volume. Neurological Research, 34, 232-237. https://doi.org/10.1179/1743132811Y.0000000080
- 27. Shoamanesh, A., Preis, S.R., Beiser, A.S., et al. (2015) Inflammatory Biomarkers, Cerebral Microbleeds, and Small Vessel Disease: Framingham Heart Study. Neurology, 84, 825-832. https://doi.org/10.1212/WNL.0000000000001279
