Journal of Organic Chemistry Research
Vol.05 No.02(2017), Article ID:20535,8
pages
10.12677/JOCR.2017.52010
Synthesis of 3,4-Dihydropyrimidine-2-(1H)- Ones/Thiones Catalyzed by Ionic Liquid [C2O2BBTA][TFA]
Zengpeng Zhang, Rong Ma, Lei Guo, Chenjiang Liu*
The Key Laboratory of Oil and Gas Fine Chemicals, Ministry of Education & Xinjiang Uygur Autonomous Region, Physics and Chemistry Detecting Center, Xinjiang University, Urumqi Xinjiang
*通讯作者。

Received: Apr. 26th, 2017; accepted: May 14th, 2017; published: May 17th, 2017

ABSTRACT
Carboxyl functional ionic liquid with benzotriazole cation and trifluoroacetate anion can be used as environmental-friendly catalyst for the efficient synthesis of 3,4-dihydropyrimidin-2(1H) ones /thiones under solvent-free conditions. Moreover, the ionic liquid [C2O2BBTA][TFA] can be easily recycled and reused for at least four cycles without obvious loss of catalytic activity.
Keywords:Ionic Liquids, Catalysis, Solvent-Free, 3,4-Dihydropyrimidin-2(1H)-Ones/Thiones
离子液体[C2O2BBTA][TFA]催化合成3,4-二氢嘧啶-2-(1H)-酮/硫酮
张增鹏,麻荣,郭磊,刘晨江*
石油天然气精细化工教育部&自治区重点实验室,新疆大学理化测试中心,新疆 乌鲁木齐

收稿日期:2017年4月26日;录用日期:2017年5月14日;发布日期:2017年5月17日

摘 要
无溶剂条件下,阳离子为苯并三唑、阴离子为三氟乙酸根的羧基功能化离子液体作为环境友好的催化剂,高效地合成了一系列3,4-二氢嘧啶-2(1H)-酮或硫酮。此外,离子液体[C2O2BBTA][TFA]循环使用至少4次,且催化活性没有明显降低。
关键词 :离子液体,催化,无溶剂,3,4-二氢嘧啶酮-2(1H)-酮/硫酮

Copyright © 2017 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
众所周知,3,4-二氢嘧啶-2(1H)-酮/硫酮化合物具有抗过敏、降压、杀菌、消炎、抗病毒 [1] - [6] 及抑制有丝分裂驱动蛋白 [7] [8] 等重要的药理和生物活性。100多年前,意大利化学家Biginelli首次提出了浓盐酸催化苯甲醛、尿素和乙酰乙酸乙酯三组分反应合成3,4-二氢嘧啶-2(1H)-酮/硫酮的方法 [9] 。然而该方法存在条件苛刻、反应时间长(18 h)且产率低(20%~50%)的缺点。因此,各种催化体系被用于该反应以改进经典方法的不足,如多组分聚合物1,4-DHP和3,4-DHPM [10] 、纳米共催化剂TiO2-SiO2 [11] 、Cu-EDTA负载的APTMS-Fe3O4@SiO2核-壳体系 [12] 、硅钛铝氧化物MxOy [13] 、微波促进 [14] 等。
离子液体因具有蒸气压低、热稳定性好、毒性低、易于回收等诸多优点,在Biginelli反应中也得到了应用 [15] [16] 。基于本课题组在离子液体合成和催化应用领域的研究基础 [17] [18] ,本文提出了一种Brønsted酸性苯并三唑离子液体催化合成3,4-二氢嘧啶-2(1H)-酮/硫酮的方法。考察了催化剂种类和用量、反应溶剂、反应时间等因素对反应产率的影响,同时对反应底物的普适性进行了研究。此外,还探讨了离子液体的催化循环使用效果。
2. 实验部分
2.1. 试剂与仪器
薄层层析硅胶用GF254硅胶,柱层析硅胶:300-400目(青岛海洋化工厂)。美国Varian inova-400型核磁共振仪(400 MHz, TMS);德国Bruker Equinox 55红外光谱仪(KBr压片);美国HP 1100液相色谱质谱仪;瑞士Büchi B-560型熔点仪。所用试剂均为市售分析纯,用前未经处理。
2.2. 离子液体的合成
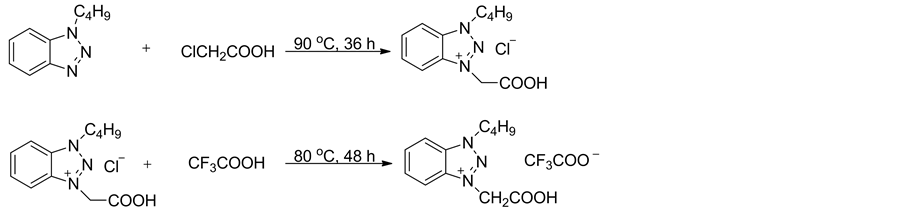
离子液体1-丁基-3-羧甲基苯并三唑三氟乙酸盐的合成如式1所示。将0.20 mol的1-丁基苯并三唑和0.24 mol的1-氯乙酸在90℃搅拌反应36 h,冷却至室温,用乙醚和丙酮(V:V = 2:1, 3 × 20 mL)混合溶剂浸泡洗涤所得的棕色固体,抽滤,所得固体在90℃下真空干燥10 h,即得氯化1-丁基-3-羧甲基苯并三唑 [19] ,白色固体,熔点:148℃~149℃。
在室温下,将0.012 mol三氟乙酸缓慢滴加到0.01 mol氯化1-丁基-3-羧甲基苯并三唑中,滴毕升温至80℃回流反应48 h,得到褐色液体,减压旋除过量的三氟乙酸,残余物在90℃下真空干燥10 h,即得离子液体1-丁基-3-羧甲基苯并三唑三氟乙酸盐[C2O2BBTA][TFA]。
离子液体[C2O2BBTA][TFA]表征数据:褐色液体,[C2O2BBTA][TFA]:1H NMR (400 MHz, DMSO) δ: 8.79-8.24 (m, 3H), 8.06-7.96 (m, 2H), 5.93 (s, 2H), 5.08 (t, J = 7.1 Hz, 2H), 2.06-1.99 (m, 2H), 1.39-1.31 (m,
Scheme 1. The synthesis of ionic liquid [C2O2BBTA][TFA]
图式1. 离子液体[C2O2BBTA][TFA]的合成
2H), 0.93 (t, J = 7.4 Hz, 3H), 13C NMR (100 MHz, DMSO) δ: 166.26 134.99, 134.22, 131.13, 130.80, 120.96, 117.76, 114.22, 113.85, 52.66, 51.09, 30.27, 18.81, 13.09. IR (KBr, ν/cm-1): 3106, 2967, 2940, 2879, 2511, 1738, 1505, 1471, 1364, 1190, 1141, 1029, 754, 718, 643, 599. ESI-MS: m/z (%) = 234.1 (100%) [M + H] +.
2.3. 未知化合物4a-4s的合成及结构分析
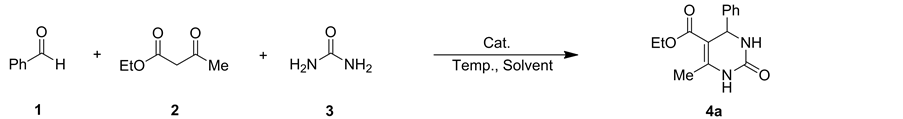
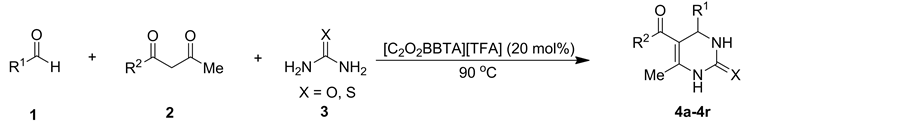
化合物4a-4r的合成反应如图式2所示。在10 mL圆底烧瓶中加入2 mmol芳香醛、2 mmol β-二羰基化合物和3 mmol脲或硫脲,20 mol%催化剂[C2O2BBTA][TFA],混合均匀后在90℃无溶剂条件下磁力搅拌反应40 min。反应结束后,向混合物中加入大量的碎冰,室温充分搅拌至碎冰融化,过滤即得产物粗品,经过柱层析分离得化合物4a-4r纯品。化合物结构经1H NMR,13C NMR,IR和MS确证结构。
目标化合物的表征如下:
4g:白色固体;1H NMR (400 MHz, DMSO-d6), δ: 9.30 (s, 1H), 7.74 (s, 1H), 7.36 (ddd, J = 15.0, 8.8, 4.4 Hz, 2H), 7.21 (d, J = 2.5 Hz, 1H), 5.60 (d, J = 2.8 Hz, 1H), 3.90 (q, J = 7.1 Hz, 2H), 2.30 (s, 3H), 1.00 (t, J = 7.1 Hz, 3H);
4h:白色固体;1H NMR (400 MHz, DMSO-d6), δ: 9.32 (s, 1H), 7.78 (s, 1H), 7.48 (dd, J = 8.8, 5.2 Hz, 1H), 7.23 – 6.92 (m, 2H), 5.59 (s, 1H), 3.91 (q, J = 7.1 Hz, 2H), 2.30 (s, 3H), 1.00 (t, J = 7.1 Hz, 3H);
4i:白色固体;1H NMR (400 MHz, DMSO) δ: 9.30 (s, 1H), 7.79 (s, 1H), 7.49 (dd, J = 6.7, 2.2 Hz, 1H), 7.35 (t, J = 8.7 Hz, 1H), 7.27 (dd, J = 4.9, 2.2 Hz, 1H), 5.15 (d, J = 3.3 Hz, 1H), 4.18 – 3.86 (m, 2H), 2.26 (s, 3H), 1.10 (t, J = 7.1 Hz, 3H);
4m:白色固体;1H NMR (400 MHz, DMSO-d6), δ: 9.15 (s, 1H), 7.70 (s, 1H), 7.24 (t, H), 6.76-6.83 (m, 3H), 5.10 (s, 1H), 4.82 (m, 1H), 3.72 (s, 3H), 2.23 (s, 3H), 1.16 (d, J = 8.0 Hz, 3H), 1.01 (d, J = 8.0, 3H);

Scheme 2. Synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones (4a-4r)
图式2. 3,4-二氢嘧啶酮/硫酮(4a-4r)的合成
4n:橙色固体;1H NMR (400 MHz, DMSO-d6), δ: 9.32 (s, 1H), 9.07 (s, 1H), 7.59 (s, 1H), 7.02 (d, J = 4.0 Hz, 2H), 6.68 (d, J = 4.0 Hz, 2H), 5.02 (s, 1H), 4.82-4.78 (m, 1H), 2.22 (s, 3H), 1.15 (d, J = 6.4 Hz, 3H), 1.00 (d, J = 6.4 Hz, 3H);
4o:绿色固体;1H NMR (400 MHz, DMSO-d6), δ: 9.05 (s, 1H), 7.56 (s, 1H), 7.03 (d, J = 4.0 Hz, 2H), 6.65 (d, J = 4.0 Hz, 2H), 5.01 (s, 1H), 4.82-4.80 (m, 1H), 2.84 (s, 6H), 2.22(s, 3H), 1.17 (d, J = 6.0 Hz, 3H), 1.03 (d, J = 6.0 Hz, 3H);
4p:淡黄色固体;1H NMR (400 MHz, DMSO-d6), δ: 9.10 (s, 1H), 7.67 (s, 1H), 7.22-7.26 (m, 2H), 7.13-7.14 (m, 2H), 5.08 (s, 1H), 2.21 (s, 3H), 1.28 (s, 9H);
4q:淡黄色固体;1H NMR (400 MHz, DMSO-d6) δ: 9.05 (d, J = 1.6 Hz, 1H), 7.64 – 7.63 (m, 1H), 7.23-7.21 (m, 1H), 7.06 – 7.02 (m, 3H), 5.07 (d, J = 3.2 Hz, 1H), 2.28 (s, 3H), 2.21 (s, 3H), 1.29 (s, 9H);
4r:淡黄色固体;1H NMR (400 MHz, DMSO-d6), δ: 9.08 (s, 1H,); 7.68 – 7.67 (m, 1H), 7.25 (t, J = 8.0 Hz, 1H), 6.83 – 6.78 (m, 3H), 5.08 (d, J = 3.2 Hz, 1H), 3.73 (s, 3H), 2.22 (s, 3H), 1.31 (s, 9H);
3. 结果与讨论
3.1. 最优反应条件的筛选
以苯甲醛、乙酰乙酸乙酯和脲三组分反应为模型,考察了催化剂种类和用量、溶剂种类、反应时间等因素对反应的影响。首先考察了2种不同阴离子的1-丁基-3-羧甲基苯并三唑离子液体及相应Brønsted酸三氟乙酸对反应的影响(表1, entries 1-3)。从表中可以看出,离子液体[C2O2BBTA][TFA]的催化活性优于离子液体[C2O2BBTA]Cl和三氟乙酸。其次,考察了催化剂的用量对反应体系的影响(表1, entries 4, 5),发现催化剂用量为20 mol%时,产物产率最高为96%。随后考察了H2O、CH3OH、C2H5OH、i-PrOH、
Table 1. Optimization of reaction conditionsa
表1. 反应条件的优化a
a反应条件:苯甲醛(2 mmol),乙酰乙酸乙酯(2 mmol),脲(3 mmol) %,90℃;b分离产率。
CH2Cl2、CH3CN、DMF、甲苯等八种溶剂及无溶剂条件下反应的效果,发现无溶剂条件下反应效果最佳(表1, entries 6-13)。最后我们对反应时间进行了筛选(表1, entries 14-17),结果表明最佳反应时间是40 min。因此,最优的反应条件为:无溶剂条件下,离子液体[C2O2BBTA][TFA](20 mol%)为催化剂,90℃反应40 min。
3.2. 底物普适性研究
在最优条件下,我们对该反应的底物普适性进行了研究,结果见表2。从中可以看出,苯甲醛的苯环上不管是带有供电子基团还是吸电子基团,都能顺利的参与反应,以82%~98%的收率得到相应的3,4-二氢嘧啶-2(1H)-酮产物(表2, entries 4a-4j)。硫脲代替脲也被用于Biginelli三组分反应,成功地合成了相应的产物(表2, entries 4k, 4l)。使用乙酰乙酸异丙酯、乙酰乙酸叔丁酯代替1,3-二羰基化合物参与反应也能得到令人满意的结果,相应产物的产率为89%~99% (表2, entries 4m-4r)。因此,离子液体1-丁基-3-羧甲基苯并三唑三氟乙酸盐催化合成二氢嘧啶-2(1H)-酮/硫酮化合物具有很好的底物普适性。
3.3. 离子液体循环使用性
离子液体的特性之一是循环使用,本文以苯甲醛、乙酰乙酸乙酯和脲三组分反应为模型,在最优条件下考察了离子液体催化剂1-丁基-3-羧甲基苯并三唑三氟乙酸盐的循环使用效果。具体方法为:将反应结束萃取分离的水相减压旋除水,残余物经真空干燥至恒重,即得回收的离子液体[C2O2BBTA][TFA],可直接用于下一次催化循环。从图1可知,离子液体催化剂1-丁基-3-羧甲基苯并三唑三氟乙酸盐循环使用4次后仍能保持较好的催化活性,表明该离子液体具有较好的循环使用效果。
Table 2. Investigation of substrate scopea
表2. 底物普适性研究a
a反应条件:芳香醛(2 mmol),1,3-二羰基化合物(2 mmol),脲或硫脲(3 mmol),[C2O2BBTA][TFA] (20 mol%),90℃,40 min;b分离产率。
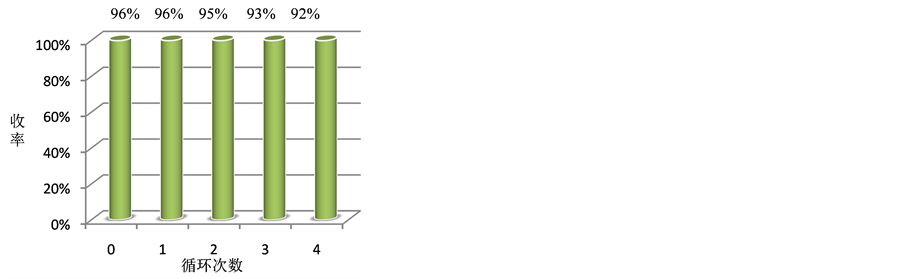
Figure 1. Recycling research of ionic liquid [C2O2BBTA][TFA]
图1. 离子液体[C2O2BBTA][TFA]的循环使用研究
4. 结论
本文发展了一种离子液体1-丁基-3-羧甲基苯并三唑三氟乙酸盐催化芳香醛、1,3-二羰基化合物和脲或硫脲绿色、高效合成3,4-二氢嘧啶-2(1H)-酮或硫酮的方法。该方法具有对环境友好、反应时间短、产率高等优点,离子液体催化剂可循环使用4次并且活性没有明显降低。
基金项目
国家自然科学基金(No. 21572195, 21262035, 21162025)。
文章引用
张增鹏,麻 荣,郭 磊,刘晨江. 离子液体[C2O2BBTA][TFA]催化合成3,4-二氢嘧啶-2-(1H)-酮/硫酮
Synthesis of 3,4-Dihydropyrimidine-2-(1H)- Ones/Thiones Catalyzed by Ionic Liquid [C2O2BBTA][TFA][J]. 有机化学研究, 2017, 05(02): 78-85. http://dx.doi.org/10.12677/JOCR.2017.52010
参考文献 (References)
- 1. Kefayati, H., Mirfarhadi, S.M. and Kazemi-Rad, R. (2015) Un-expected One-Pot Synthesis of Novel 2-Aminopyrimi- dine-4-Ones under Microwave Irradiation. Journal of the Chemistry Communication, 62, 107-111. https://doi.org/10.1002/jccs.201400248
- 2. Mohammadi, K., Shirini, F. and Yahyazadeh, A. (2015) 1,3-Disulfonic Acid Imidazolium Hydrogen Sulfate: A Reusable and Efficient Ionic Liquid for the One-Pot Mul-ti-Component Synthesis of Pyrimido[4,5-b]Quinoline Derivatives. RSC Advances, 5, 23586-23590. https://doi.org/10.1039/C5RA02198G
- 3. Elhamifar, D., Nasr-Esfahani, M, Karimi, B., Moshkelgosha, R. and Shábani, A. (2016) Ionic Liquid and Sulfonic Acid Based Bifunctional Periodic Mesoporous Organosilica (BPMO-IL-SO3H) as a Highly Efficient and Reusable Nanocatalyst for the Biginelli Reaction. ChemCatChem, 6, 2593-2599.
- 4. Zhang, Y.H., Wang, B., Zhang, X.M., Huang, J.B. and Liu, C.J. (2015) An Efficient Synthesis of 3,4-Dihydropyrimi- din-2(1H)-Ones and Thiones Catalyzed by a Novel Brønsted Acidic Ionic Liquid under Solvent-Free Conditions. Molecules, 20, 3811-3820. https://doi.org/10.3390/molecules20033811
- 5. Li, H., Liu, C., Zhang, Y., Sun, Y., Wang, B. and Liu, W. (2015) Green Method for the Synthesis of Chromeno[2,3-c]- Pyrazol-4(1H)-Ones through Ionic Liquid Promoted Directed Annulation of 5-(Aryloxy)-1H-Pyrazole-4-Carbalde- hydes in Aqueous Media. Organic Letters, 17, 932-935. https://doi.org/10.1021/acs.orglett.5b00033
- 6. Xue, F., Ma, R., Sun, Y., Abdukader, A., Zhang, Y. and Liu, C. (2015) Syntheses of Carboxyl Functionalized Benzotriazol-Based Ionic Liquids and Their Application in Extraction-Oxidative Desulfurization. Chemical Journal of Chinese Universities, 36, 1298-1303.
- 7. Atwalk, K.S., Rovnyak, G.C., Kimball, S.D., et al. (1990) Dihydropyrimidine Calcium Channel Blockers. II. 3-Sub- stituted-4-Aryl-1,4-Dihydro-6-Methyl-5-Pyrimidinecarboxylic Acid Esters as Potent Mimics of Dihydropyridines. Journal of Medicinal Chemistry, 33, 2629-2635. https://doi.org/10.1021/jm00171a044
- 8. Rao, G.B.D., Acharya, B.N., Verma, S.K. and Kaushik, M.P. (2011) N,N’-Dichlorobis(2,4,6-Trichlorophenyl)Urea (CC-2) as a New Reagent for the Synthesis of Pyrimidone and Pyrimidine Derivatives via Biginelli Reaction. Tetrahedron Letters, 52, 809-812.
- 9. Yadav, J.S., Reddy, B.V.S., Sridhar, P., Reddy, J.S.S., Nagaiah, K., Lingaiah, N. and Saiprasad, P.S. (2004) Green Protocol for the Biginelli Three-Component Reaction: Ag3PW12O40 as a Novel, Water-Tolerant Heteropolyacid for the Synthesis of 3,4-Dihydropyrimidinones. European Journal of Organic Chemistry, 2004, 552-557. https://doi.org/10.1002/ejoc.200300559
- 10. Li, W., Zhou, G., Zhang, P., Lai, Y. and Xu, S. (2011) One-Pot Synthesis of Dihydropyrimidiones via Environmentally Friendly Enzyme-Catalyzed Biginelli Reaction. Heterocycles, 83, 2067-2077. https://doi.org/10.3987/COM-11-12267
- 11. Gholap, A.R., Venkatesan, K., Daniel, T., Lahoti, R.J. and Srinivasan, K.V. (2004) Ionic Liquid Promoted Novel and Efficient One Pot Synthesis of 3,4-Dihydropyrimidin-2-(1H)-Ones at Ambient Temperature under Ultrasound Irradiation. Green Chemistry, 6, 147-150. https://doi.org/10.1039/b314015f
- 12. Da Silva, D.L., Fernandes, S.A., Sabino, A.A. and De Fatima, A. (2011) p-Sulfonic Acid Calixarenes as Efficient and Reusable Organocatalysts for the Synthesis of 3,4-Dihydropyrimidin-2(1H)-Ones/-Thiones. Tetrahedron Letters, 52, 6328-6330.
- 13. Khabazzadeh, H., Saidi, K. and Sheibani, H. (2008) Microwave-Assisted Synthesis of Dihydropyrimidin-2(1H)-Ones Using Graphite Supported Lanthanum Chloride as a Mild and Efficient Catalyst. Bioorganic & Medicinal Chemistry Letters, 18, 278-280.
- 14. Kappe, C.O. (1993) 100 Years of the Biginelli Dihydropyrimidine Synthesis. Tetrahedron, 49, 6937-6963.
- 15. Overman, L.E., Rabinowitz, M.H. and Renhowe, P.A. (1995) Enantioselective Total Synthesis of (-)-Ptilomycalin A. Journal of the American Chemical Society, 117, 2657-2658. https://doi.org/10.1021/ja00114a034
- 16. Chitra, S. and Pandiarajan, K. (2009) Calcium Fluoride: An Efficient and Reusable Catalyst for the Synthesis of 3,4-Dihydropyrimidin-2(1H)-Ones and Their Corresponding 2(1H)Thione: An Improved High Yielding Protocol for the Biginelli Reaction. Tetrahedron Letters, 50, 2222-2224.
- 17. Snider, B.B., Chen, J., Patil, A.D. and Freyer, A.J. (1996) Synthesis of the Tricyclic Portions of Batzelladines A, B and D. Revision of the Stereochemistry of Batzelladines A and D. Tetrahedron Letters, 37, 6977-6980.
- 18. Kappe, C.O., Fabian, W.M.F. and Semones, M.A. (1997) Conformational Analysis of 4-Aryl-Dihydropyrimidine Calcium Channel Modulators. A Comparison of ab Initio, Semiempirical and X-Ray Crystallographic Studies. Tetrahedron, 53, 2803-2816.
- 19. Yang, Z.Y. and Guo, H.Y. (2011) One-Pots Synthesis of 3,4-Dihydropyrimidin-2-(1H)-Ones Catalyzed by Acidic Ionic Liquid under Solvent-Free Conditions. Journal of Zhejiang University of Technology, 39, 511-515.
- 20. Mayer, T.U., Kapoor, T.M., Haggarty, S.J., et al. (1999) Small Molecule Inhibitor of Mitotic Spindle Bipolarity Identified in a Phenotype-Based Screen. Science, 286, 971-974. https://doi.org/10.1126/science.286.5441.971
- 21. Deres, K., Schröder, C.H., Paessens, A., et al. (2003) Inhibition of Hepatitis B Virus Replication by Drug-Induced Depletion of Nucleocapsids. Science, 299, 893-896. https://doi.org/10.1126/science.1077215
- 22. Li, X., Liu, C, Wang, J. and Li, Y. (2009) Lanthanum Nitrate as an Efficient Catalyst for the Synthesis of 3,4-Dihydro- pyrimidine-2(1H)-(Thio)Ones. Chemistry, 9, 837-840.
- 23. Wu, H., Fu, C., Zhao, Y., Yang, B., Wei, Y., Wang, Z. and Tao, L. (2015) Multicomponent Copolycondensates via the Simultaneous Hantzsch and Biginelli Reactions. ACS Macro Letters, 4, 1189-1193. https://doi.org/10.1021/acsmacrolett.5b00637
- 24. Titova, Y., Fedorova, O., Rusinov, G., Vigorov, A., Krasnov, V., Murashkevich, A. and Charushin, V. (2015) Effect of Nanosized TiO2-SiO2 Covalently Modified by Chiral Mole-cules on the Asymmetric Biginelli Reaction. Catalysis Today, 241, 270-274.
- 25. Sheykhan, M., Yahyazadeh, A. and Rahemizadeh, Z. (2016) Cu-EDTA-Modified APTMS-Fe3O4@SiO2 Core-Shell Nanocatalyst: A Novel Magnetic Re-coverable Catalyst for the Biginelli Reaction. RSC Advances, 6, 34553-34563. https://doi.org/10.1039/C6RA02415G
- 26. Fedorova, O.V., Titova, Y.A., Vigorov, A.Y., Toporova, M.S., Alisienok, O.A., Murashkevich, A.N., Krasnov, V.P., Rusinov, G.L. and Charushin, V.N. (2016) Asymmetric Biginelli Reaction Catalyzed by Silicon, Titanium and Aluminum Oxides. Catalysis Letters, 146, 493-498. https://doi.org/10.1007/s10562-015-1666-5
