Applied Physics
Vol.06 No.11(2016), Article ID:19089,7
pages
10.12677/APP.2016.611033
Phase Transition and Magnetic Properties in the Forming Process of Sc Doped M-Type Hexaferrite
Hao Zhou, Rujun Tang*
Jiangsu Key Laboratory of Thin Films, College of Physics, Optoelectronics and Energy, Soochow University, Suzhou Jiangsu

Received: Nov. 9th, 2016; accepted: Nov. 23rd, 2016; published: Nov. 29th, 2016
Copyright © 2016 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



ABSTRACT
Sc doped M-type hexaferrite BaFe10.2Sc1.8O19 was prepared with the conventional solid state reaction method. The phase and magnetic property transition processes in the sintering process of M-type hexaferrite BaFe10.2Sc1.8O19 have been investigated. Results show that the spinel phase BaFe2O4 and ion-deficient M-type hexaferrite dominate when sintering temperature is lower than 1000˚C. The M-type hexaferrite BaFe10.2Sc1.8O19 appears above 1050˚C and does not become a single phase until 1200˚C. Both the remanence ratio and coercivity of the samples decrease with increasing sintering temperature and drop to nearly zero above 1200˚C. However, the saturation magnetization of the samples increases with increasing sintering temperature until 1050˚C and then decreases. The magnetic property transition process agrees well with the phase transition process. The above results show that incorporation of Sc3+ into the M-type hexaferrite needs a higher thermal energy than that of Fe3+, and will lead to a drastic change in the magnetic properties of M-type hexaferrite.
Keywords:M-Type Hexaferrite, Sc3+ Substitution, Sintering Temperature, Phase Transition, Magnetic Property

Sc掺杂M型钡铁氧体烧结过程中的相变 及磁性研究
周浩,汤如俊*
苏州大学物理与光电·能源学部,江苏省薄膜材料重点实验室,江苏 苏州

收稿日期:2016年11月9日;录用日期:2016年11月23日;发布日期:2016年11月29日

摘 要
本文采用固相反应法制备了掺杂M型钡铁氧体BaFe10.2Sc1.8O19,并对其在烧结过程中的相变及磁性质做了一定的研究。结果表明,当烧结温度低于1000℃时,尖晶石相BaFe2O4和铁离子缺失的M型铁氧体是主要的烧结产物。当烧结温度高于1050℃时,M型铁氧体BaFe10.2Sc1.8O19开始生成。直到当烧结温度达到1200℃时,BaFe10.2Sc1.8O19才以单相的形式存在于烧结产物中。随着烧结温度的升高,烧结产物的剩磁比和矫顽场逐步降低,并在烧结温度高于1200℃的时候降到接近于零。饱和磁化强度随着烧结温度的升高逐步升高,并在1050℃的时候达到最大值,随后逐渐降低。磁性质的变化过程与烧结产物的相变是一致的。以上结果表明,相对于Fe3+,Sc3+对M型铁氧体的掺杂需要更高的热能,并会导致M型铁氧体磁性质的较大变化。
关键词 :M型铁氧体,Sc3+掺杂,烧结温度,相变,磁性质

1. 引言
M型铁氧体BaFe12O19(以下简称BaM)是六角铁氧体的一种 [1] 。BaM的晶体结构可以看成是SRS*R*形式的交替堆叠。其中,S代表BaFe2O4,R代表(BaFe6O11)2−,S*、R*分别表示由S、R绕c轴作180°旋转所得。相对于尖晶石铁氧体,BaM沿c轴表现出各向异性,并拥有更高的磁晶各向异性常数(K1 = 3.3 × 105 J/m3) [2] 。因而,在过去的几十年里,BaM被广泛应用于永磁体以及高密度存储器等等 [3] [4] [5] [6] 。除此之外,其在GHz频段微波器件方面也有潜在的应用前景。其次,掺入不同的离子对BaM的磁性会带来不同改变,这也进一步拓展了BaM在其他方面的应用 [7] 。比如,Al和Ga的掺杂有利于提高BaM的磁各向异性常数 [8] [9] [10] ,而其他诸如In和Co-Ti的掺杂却能够降低BaM的磁各向异性常数 [11] [12] [13] [14] 。据文献报道,Sc3+对BaM中Fe3+的少量取代也会使其各向异性常数显著降低,大小约为零,而相应的晶体结构却不会发生改变 [15] 。Sc3+掺杂对BaM磁结构和各向异性的巨大改变,使其转变为一种软磁性材料 [16] 。进一步的研究表明,钪的掺杂会带来材料磁结构的改变,从而使Sc3+掺杂BaM(以下简称ScBaM)拥有磁电耦合性质,这也奠定了其可能应用于一些新型器件的基础 [17] 。然而,直到现在,Sc3+掺杂对BaM合成过程中的磁性质的影响还没有被系统研究过。在科学研究以及工业生产中,固相反应法已经被广泛应用于多晶氧化物材料的合成中 [18] [19] 。同样,固相反应法也是合成多晶M型铁氧体的最好方法之一 [2] 。对ScBaM固相烧结过程中相变的系统研究将有助于对其磁性质的变化有一个更深入、更系统的理解。本工作中,我们将系统地研究Sc3+掺杂M型钡铁氧体BaFe10.2Sc1.8O19烧结过程中的相变以及磁性质的改变。
2. 实验方法
本文采用固相反应法制备了Sc3+掺杂M型六角铁氧体BaFe10.2Sc1.8O19。高纯度(99.99%)的BaCO3,Sc2O3和Fe2O3粉末按照化学计量比配料,混合球磨24个小时。将球磨所得混合物干燥,再在不同温度下(950℃~1250℃)烧结10个小时,烧结气氛为空气,之后按1℃/min降至100℃,随后自然降温。X射线衍射仪(XRD,型号为Rigaku D/Max 2000PC)用来分析样品相结构,扫描电子显微镜(SEM,型号为Hitachi,SU8010)用来分析样品的显微形貌,综合物性测量仪(PPMS,型号为PPMS-9,Quantum Design)上集成的振动样品磁强计(VSM)用来测量样品的磁性质。
3. 结果与分析
图1是不同烧结温度条件下所得产物的XRD衍射图。从图中可以看出,当烧结温度为950℃时,烧结产物包含四相:Fe2O3,Sc2O3,BaFe2O4和缺铁的M型钡铁氧体(图中“+”峰位代表的产物)。这个缺铁的M型钡铁氧体可能是BaFexO19 (10.2 ≤ x < 12)或者BaFexScyO19 (10.2 ≤ x < 12, 0 ≤ y < 1.8)。该XRD峰在1150℃以上突然消失表明BaFexO19比BaFexScyO19更有可能。此外,根据文献报道,铁离子缺失前驱体对单相M型钡铁氧体的形成有重要意义,烧结产物中M型铁氧体BaFexO19(10.2 ≤ x ≤ 12)的产生很有可能源于这种情况 [20] [21] [22] [23] 。包含Fe2O3的多相产物表明固相反应还没有结束。在1000℃烧结温度下,Fe2O3的峰强降低而BaFexO19的峰强增强,说明M型铁氧体BaFexO19得到进一步结晶。当烧结温度达到1050℃时,Fe2O3和BaFe2O4的峰消失,Sc2O3的峰也几近消失,伴随着一些新的峰出现在BaFexO19峰位的左侧。随着烧结温度的升高,BaFexO19的峰强逐渐降低,新出现峰的峰强逐渐增强,但峰位没有明显变化。当烧结温度达到1200℃及以上时,只能观察到左侧新出现的峰,说明了固相反应的完成。所以,考虑到Sc的原子半径大于Fe,可以认为左侧新出的峰就是M型钡铁氧体BaFe10.2Sc1.8O19的峰。
图2是1250℃烧结温度下所得BaFe10.2Sc1.8O19单相粉末的XRD衍射图。根据衍射图可以计算出烧结所得多晶BaFe10.2Sc1.8O19材料的晶格常数为:a = b = 5.94Å,c = 23.67Å。计算所得晶格常数和文献中
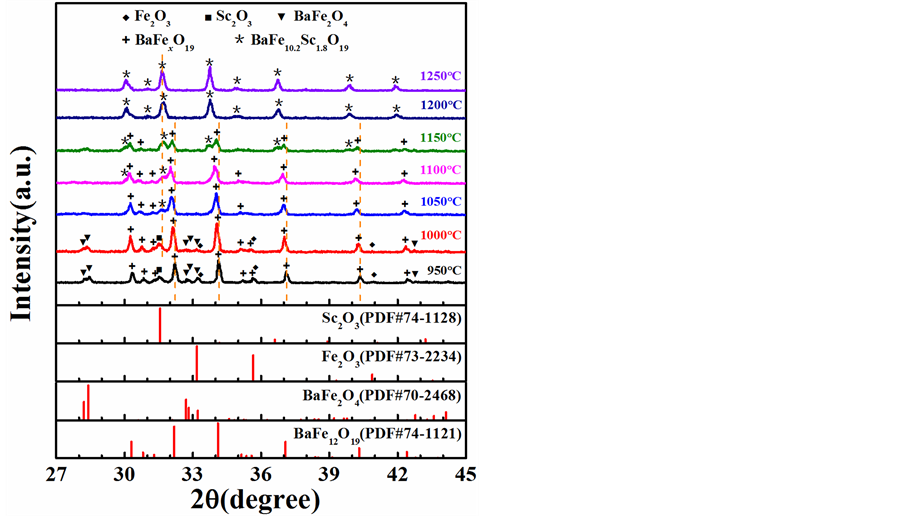
Figure 1. The XRD spectra of powder samples with the same stoichiometric proportion as BaFe10.2Sc1.8O19 but under different sintering temperatures
图1. 按相同化学计量(BaFe10.2Sc1.8O19)配比粉末在不同烧结温度条件下所得产物的XRD衍射图
报道的单晶BaFe10.2Sc1.8O19材料的晶格常数(a = b = 5.96 Å, c = 23.61 Å)较为接近,并且稍大于单晶BaFe12O19材料(a = b = 5.89 Å, c = 23.18 Å) [15] [24] 。以上结果结合图1,可以证实从1050℃烧结温度开始,图1中新出现的峰就是M型钡铁氧体BaFe10.2Sc1.8O19的峰。进一步说明,相对于M型钡铁氧体BaFe12O19,BaFe10.2Sc1.8O19的形成需要更高的烧结温度。这可能由于相对于Fe,更大原子半径的Sc要掺入M型钡铁氧体中需要破除更高的能量壁垒。
图3是不同烧结温度所得样品的扫描电镜照片。可以看到,当烧结温度在1050℃及以下时,样品晶粒尺寸没有明显变化。当烧结温度高于1050℃时,样品晶粒尺寸随烧结温度的升高而增大。在烧结温度达到1150℃时,就已经可以看到明显的六角晶体结构。
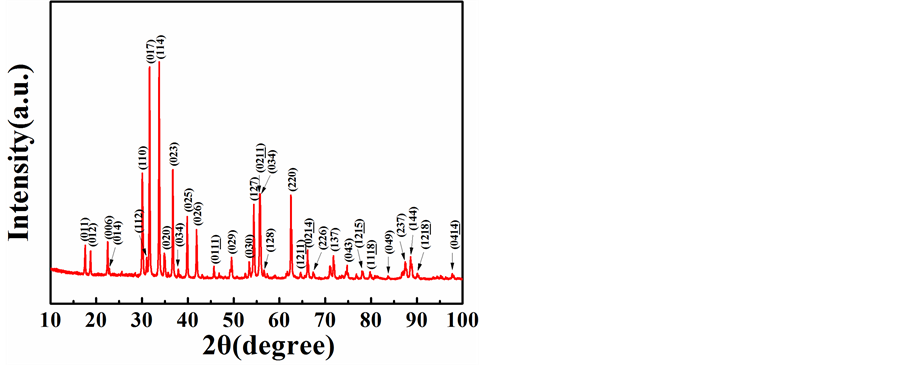
Figure 2. XRD spectrum of BaFe10.2Sc1.8O19 powder sintered at 1250˚C
图2. 1250℃烧结温度下所得BaFe10.2Sc1.8O19粉末的XRD衍射图
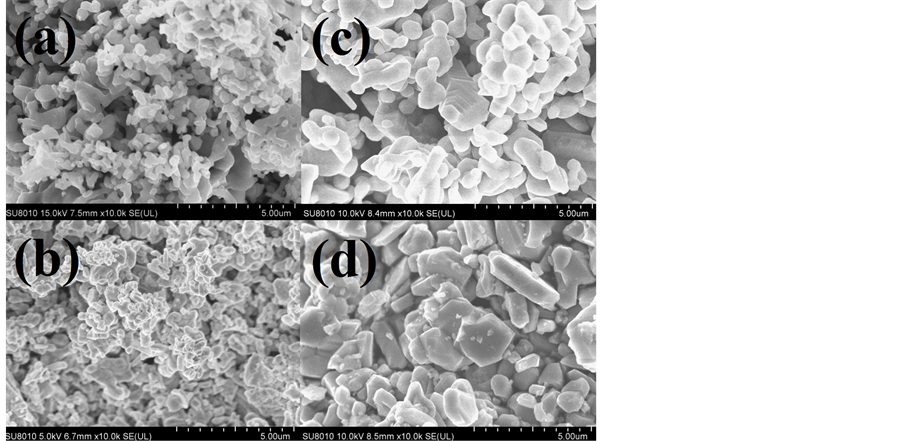
Figure 3. SEM images of the samples sintered under the temperatures of (a) 950˚C, (b) 1050˚C, (c) 1150˚C and (d) 1250˚C
图3. (a) 950℃,(b) 1050℃,(c) 1150℃和(d) 1250℃烧结温度下烧结所得样品的扫描电镜照片
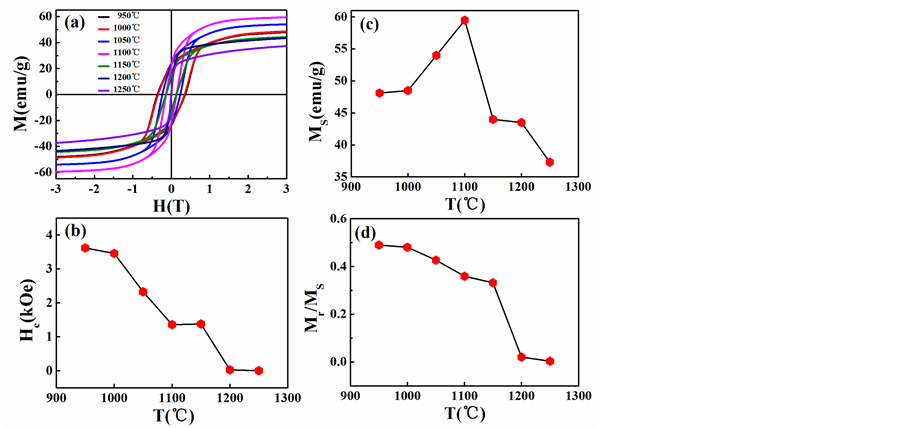
Figure 4. The temperature dependence of room temperature (a) magnetic hysteresis loops, (b) coercive field (Hc), (c) saturation magnetic moment (Ms) and (d) remanence ratios (Mr/Ms) for powder samples sintered at different temperatures
图4. 室温下,不同烧结温度所得样品的(a)磁滞回线,(b)矫顽场(Hc),(c)饱和磁化强度(Ms),(d)剩磁比(Mr/Ms)随烧结温度的变化图
图4是室温下,烧结所得样品的磁性质随烧结温度的变化图。从图4(a)可以看出,样品的磁滞回线随烧结温度的变化发生了明显的改变。图4(b)表明样品的矫顽场(Hc)随烧结温度的升高而逐渐降低。具体的,当烧结温度低于1000℃时,Hc大于3000Oe,这也说明了此烧结温度下,硬磁材料BaFexO19 (10.2 ≤ x ≤ 12)是主要的烧结产物。当烧结温度高于1200℃时,Hc快速下降到约为0Oe,说明了固相反应的结束。M型铁氧体BaFe10.2Sc1.8O19和BaFexO19 (10.2 ≤ x ≤ 12)拥有类似的晶体结构,但磁结构却有很大差异。M型铁氧体BaFe10.2Sc1.8O19的磁晶各向异性常数接近于零,因此表现出软磁性。图4(c)表明样品的饱和磁化强度(Ms)开始随烧结温度的升高而逐渐增大,在1100℃时达到一个最大值,并在之后随烧结温度的升高而急剧降低。由于总的磁性原子数未发生改变,所以磁性的变化应该归因于烧结产物的相变。那么可以认为,Ms开始的增大是源于BaFexO19 (10.2 ≤ x ≤ 12)的生成,而之后的降低则是由于BaFe10.2Sc1.8O19的产生。有文献报道,非磁性Sc3+对Fe3+的取代会影响M型铁氧体中S、R、S*、R*界面处铁原子之间的交换作用,从而能够导致Ms的降低 [25] [26] 。Ms在1100℃时出现峰值更进一步说明了烧结温度在1050℃以上时,BaFe10.2Sc1.8O19开始形成。图4(d)表明样品的剩磁比(Mr/Ms)随烧结温度的升高而逐渐降低,其变化趋势和Hc相类似,进一步说明了M型铁氧体在Sc掺杂前后由硬磁性材料向软磁性材料的转变。
4. 总结
本文采用固相反应法制备了Sc3+掺杂M型钡铁氧体BaFe10.2Sc1.8O19,并对其在烧结过程(950℃~1250℃)中的相变及磁性质做了一定的研究。研究表明,当烧结温度低于1000℃时,尖晶石相BaFe2O4和离子缺失的M型铁氧体BaFexO19(10.2 ≤ x ≤ 12)是主要的烧结产物。当烧结温度高于1050℃时,Sc3+掺杂M型铁氧体BaFe10.2Sc1.8O19开始生成。直到当烧结温度达到1200℃时,BaFe10.2Sc1.8O19才以单相的形式存在于烧结产物中。随着烧结温度的升高,烧结产物的剩磁比和矫顽场逐步降低,并在烧结温度高于1200℃的时候降到接近于零。饱和磁化强度随着烧结温度的升高逐步升高,并在1050℃的时候达到最大值,随后逐渐降低。磁性质的变化过程与烧结产物的相变是一致的。磁性质的变化过程可以归因于烧结产物的相变以及Sc3+对M型铁氧体中Fe3+的取代。其中,Sc3+对Fe3+的取代会影响M型铁氧体中S、R、S*、R*界面处铁原子之间的交换作用,宏观上变现为Sc3+掺杂M型铁氧体磁性质的较大变化。
基金项目
本论文感谢国家自然科学基金(51202153,11274237)的支持;感谢教育部基金(20123201120028)及苏州大学的支持。
文章引用
周 浩,汤如俊. Sc掺杂M型钡铁氧体烧结过程中的相变及磁性研究
Phase Transition and Magnetic Properties in the Forming Process of Sc Doped M-Type Hexaferrite[J]. 应用物理, 2016, 06(11): 265-271. http://dx.doi.org/10.12677/APP.2016.611033
参考文献 (References)
- 1. Went, J.J., Rathenau, G.W., Gorter, E.W., et al. (1952) Ferroxdure, A Class of New Permanent Magnet Materials. Philips Technical Review, 13, 194.
- 2. Smit, J. and Wijn, H.P.J. (1959) Ferrites. Philips Technical Library, Eindhoven, The Netherlands.
- 3. Guan, Y.J., Lin, Y.B., Zou, L.Y., et al. (2013) The Effects of Co-Ti Co-Doping on the Magnetic, Electrical, and Magnetodielectric Behaviors of M-Type Barium Hexaferrites. AIP Advances, 3, 122115. https://doi.org/10.1063/1.4860948
- 4. Sugimoto, M. (1999) The Past, Present, and Future of Ferrites. Journal of the American Ceramic Society, 82, 269-280. https://doi.org/10.1111/j.1551-2916.1999.tb20058.x
- 5. Harris, V.G., Chen, Z.H., Chen, Y.J., et al. (2006) Ba-Hexaferrite Films for Next Generation Microwave Devices. ChemInform, 37. https://doi.org/10.1002/chin.200637192
- 6. Xu, P., Han, X. and Wang, M. (2007) Synthesis and Magnetic Properties of BaFe12O19 Hexaferrite Nanoparticles by a Reverse microemulsion Technique. The Journal of Physical Chemistry C, 111, 5866-5870. https://doi.org/10.1021/jp068955c
- 7. Harris, V.G., Geiler, A., Chen, Y.J., et al. (2009) Recent Advances in Processing and Applications of Microwave Ferrites. Journal of Magnetism and Magnetic Materials, 321, 2035-2047. https://doi.org/10.1016/j.jmmm.2009.01.004
- 8. Van Uitert, L.G. and Swanekamp, F.W. (1957) Permanent Magnet Oxides Containing Divalent Metal Ions. II. Journal of Applied Physics, 28, 482. https://doi.org/10.1063/1.1722776
- 9. Mones, A.H. and Banks, E. (1958) Cation Substitutions in BaFe12O19. Journal of Physics and Chemistry of Solids, 4, 217-222. https://doi.org/10.1016/0022-3697(58)90119-7
- 10. Haneda, K. and Kojima, H. (1973) Magnetization Reversal Process in Chemically Precipitated and Ordinary Prepared BaFe12O19. Japanese Journal of Applied Physics, 12, 355. https://doi.org/10.1143/JJAP.12.355
- 11. Batlle, X., Obradors, X., Rodríguez-Carvajal, J., et al. (1991) Cation Distribution and Intrinsic Magnetic Properties of Co-Ti-Doped M-Type Barium Ferrite. Journal of Applied Physics, 70, 1614-1623.
- 12. Cho, H.-S. and Kim, S.-S. (1999) M-Hexaferrites with Planar Magnetic Anisotropy and Their Application to High- Frequency Microwave Absorbers. IEEE Transactions on Magnetics, 35, 3151-3153.
- 13. Albanese, G. and Deriu, A. (1979) Magnetic properties of Al, Ga, Sc, In Substituted Barium Ferrites: A Comparative Analysis. Ceramurgia International, 5, 3-10. https://doi.org/10.1016/0390-5519(79)90002-4
- 14. Kakizaki, K., Hiratsuka, N. and Namikawa, T. (1997) Fine Structure of Acicular BaCoxTixFe12-2xO19 Particles and Their Magnetic Properties. Journal of Magnetism and Magnetic Materials, 176, 36-40. https://doi.org/10.1016/S0304-8853(97)00634-3
- 15. Perekalina, T.M. and Cheparin, V.P. (1968) Ferrimag-netism of Hexagonal Ferrites. Soviet Physics: Solid State, 9, 2524.
- 16. Mallick, K.K., Shepherd, P. and Green, R.J. (2007) Magnetic Properties of Cobalt Substituted M-Type Barium Hexaferrite Prepared by Co-Precipitation. Journal of Magnetism and Magnetic Materials, 312, 418-429. https://doi.org/10.1016/j.jmmm.2006.11.130
- 17. Tokunaga, Y., Kaneko, Y., Okuyama, D., et al. (2010) Multiferroic M-Type Hexaferrites with a Room-Temperature Conical State and Magnetically Controllable Spin Helicity. Physical Review Letters, 105, Article ID: 257201. https://doi.org/10.1103/PhysRevLett.105.257201
- 18. Sakai, T., Chen, Y., Chinnasamy, C.N., Vittoria, C. and Harris, V.G. (2006) Textured Sc-Doped Barium-Ferrite Compacts for Microwave Applications below 20 GHz. IEEE Transactions on Magnetics, 42, 3353-3355. https://doi.org/10.1109/TMAG.2006.879639
- 19. Chen, Y., Nedoroscik, M.J., Geiler, A.L., Vittoria, C. and Harris, V.G. (2008) Perpendicularly Oriented Polycrystalline BaFe11.1Sc0.9O19 Hexaferrite with Narrow FMR Linewidths. Journal of the American Ceramic Society, 91, 2952-2956. https://doi.org/10.1111/j.1551-2916.2008.02578.x
- 20. Wang, L. and Zhang, Q. (2009) Effect of Fe3+/Ba2+ Mole Ratio on the Phase Formation and Microwave Properties of BaFe12O19 Prepared by Citrate-EDTA Complexing Method. Journal of Alloys and Compounds, 469, 251-257. https://doi.org/10.1016/j.jallcom.2008.01.122
- 21. Hessien, M.M., Rashad, M.M. and El-Barawy, K. (2008) Controlling the Composition and Magnetic Properties of Strontium Hexaferrite Synthesized by Co-Precipitation method. Journal of Magnetism and Magnetic Materials, 320, 336-343. https://doi.org/10.1016/j.jmmm.2007.06.009
- 22. Rashad, M.M., Radwan, M. and Hessien, M.M. (2008) Effect of Fe/Ba Mole Ratios and Surface-Active Agents on the Formation and Magnetic Properties of Co-Precipitated Barium Hexaferrite. Journal of Alloys and Compounds, 453, 304-308. https://doi.org/10.1016/j.jallcom.2006.11.080
- 23. Zhang, W., Bai, Y., Han, X., et al. (2013) Phase Formation, Sintering Behavior and Magnetic Property of Bi-Co-Ti Substituted M-Type Barium Hexaferrite. Journal of Alloys and Compounds, 556, 20-25. https://doi.org/10.1016/j.jallcom.2012.12.113
- 24. Obradors, X., Collomb, A., Pernet, M., Samaras, D. and Joubert, J.C. (1985) X-Ray Analysis of the Structural and Dynamic Properties of BaFe12O19 Hexagonal Ferrite at Room Temperature. Journal of Solid State Chemistry, 56, 171-181. https://doi.org/10.1016/0022-4596(85)90054-4
- 25. Röschmann, P., Lemke, M., Tolksdorf, W. and Welz, F. (1984) Anisotropy Fields and FMR Linewidth in Single- Crystal Al, Ga and Sc Substituted Hexagonal Ferrites with M Structure. Materials Research Bulletin, 19, 385-392. https://doi.org/10.1016/0025-5408(84)90181-8
- 26. Dionne, G.F. and Fitzgerald, J.F. (1991) Magnetic Hysteresis Properties of BaFe12−xInxO19 Ceramic Ferrites with C-Axis Oriented Grains. Journal of Applied Physics, 70, 6140-6142. https://doi.org/10.1063/1.350022