Material Sciences
Vol.07 No.02(2017), Article ID:20045,7
pages
10.12677/MS.2017.72024
Study on Hydrophobic Coating Prepared by Plasma-Spraying Anticorrosion Performance
Liang Yang, Yang Cao*
College of Materials and Chemical Engineering, Hainan University, Haikou Hainan

Received: Mar. 14th, 2017; accepted: Mar. 27th, 2017; published: Mar. 30th, 2017

ABSTRACT
Purpose of this paper was to prepare Al2O3-TiO2 coating on Q235 substrate that has good hydrophobic nature and outstanding corrosion resistance. The Al2O3-TiO2 coating with Ni-Fe intermediate layer were prepared on Q235 alloy surface using plasma spraying technology, and then thecoating is characterized by using SEM (scanning electron microscope), contact angle measurement, salt fog corrosion chamber and electro chemical work station to measure the surface morphology, contact angle corrosion resistance and impedance spectroscopy of coating. Coating prepared by plasma spraying and dipping in the aqueous solutions of dodecanoic acid(ASD) for processing can make the static contact angle increase to 129˚, the change in surface morphology for ASD is not obvious after immersing in the nature sea water and storing in salt fog conditions. This indicated good corrosion resistance of the ASD coating.
Keywords:Anticorrosion, Resistance Hydrophobic, Plasma Spraying Technology, Static Contact Angle, Impedance Spectroscopy
等离子喷涂制备疏水涂层的防腐性能研究
杨亮,曹阳*
海南大学材料与化工学院,海南 海口

收稿日期:2017年3月14日;录用日期:2017年3月27日;发布日期:2017年3月30日

摘 要
本论文的目的是在Q235基底上制备具有好的疏水性及优秀防腐性的Al2O3和TiO2复合涂层。采用等离子喷涂技术在Q235基底上先喷NiFe中间过度层然后制备Al2O3和TiO2复合涂层并通过扫描电子显微镜、接触角测量仪、盐雾腐蚀箱和电化学工作站测量该复合涂层的表面形貌、接触角值、防腐蚀性和阻抗谱。利用等离子喷涂制备涂层并浸入到十四酸的溶液中进行处理,使涂层表面的静态接触角提高到129˚,利用盐雾腐蚀实验处理和自然海水浸泡后该涂层表面未发现明显的改变,因此,经十四酸处理的疏水涂层具有很好的防腐效果。
关键词 :防腐,疏水,等离子喷涂,静态接触角,阻抗谱

Copyright © 2017 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
结构材料或表面绝大部分自然破坏都直接或间接源自环境腐蚀作用,海水由于含有大量的盐分及微生物而导致材料损坏,仪器操作失灵、巨大经济损失甚至生命危险 [1] 。迄今为止,批量制造出完全不受海水或盐雾腐蚀的工程材料难度大、成本高且大范围应用困难,所以材料表面防护技术显的格外重要且十分有效 [2] 。目前较成熟有效的在材料表面制备保护涂层技术方法如:玻璃涂层 [3] 、Ni基涂层 [4] [5] [6] 、陶瓷涂层 [7] [8] [9] [10] 、超疏水涂层 [11] [12] 等。氧化物陶瓷层已经应用在防腐材料方面,如:TiO2 [13] 、SiO2 [14] 、Al2O3 [15] [16] 、ZrO2 [17] 及其混合物 [18] 应用效果良好,具有十分可观的应用前景。超疏水表面因其存在诱人的理论研究价值和潜在的实际应用前景而倍受研究者关注 [19] ,设计或制备超疏水的自清洁表面以期望在海洋防污、防腐 [20] 、自清洁、海运、管道运输等方面进行大规模应用。受此思路的启发,我们尝试将超疏水膜及无机陶瓷涂层相结合,应用到海洋防腐防污领域,探索具有超疏水表面防海水腐蚀的有效涂层。
本试验所用基体为厚度为2 mm的长宽10 mm普通Q235钢板,通过线切割机床和喷砂机等设备对铁片进行预处理,利用等离子喷涂技术,在样品表面制备一层致密的氧化物陶瓷层,利用十四酸进行处理,制备具有一定疏水性的陶瓷涂层,并对涂层进行耐海水腐蚀、耐盐雾腐蚀和电化学性能测试,通过SEM、金相显微检测、接触角与表面能测量分析,尝试制备具有优秀防腐防污性能的物理涂层,提高我国海防能力。
2. 试验
加速腐蚀试验
首先将Q235钢板利用线切割机加工成所需要样品尺寸10 mm × 10 mm × 2 mm的规则铁片,再用喷砂机对样品进行喷砂处理,除去其表面的污垢锈迹等,同时钢材表面也进行了粗化处理;
在多次预实验基础上确定最优化的电流、电压、送粉量等参数,实验所用参数列于表1中,利用等
Table 1. Experiments of plasma spraying parameters
表1. 实验中等离子喷涂参数
离子体将Q235基底预处理加热以消除界面热应力,然后喷涂铁镍过渡层后喷涂AT13涂层(TiO2:Al2O3 = 13%:87%)。以提高AT13与基底的结合力,提高样品的涂层的寿命。如图1所示,样品剖面图中显示二界面均相对结合紧密,未发现出现中空或脱皮现象。将制备成功的样品自然冷却到温室后分别装入样品袋中待用;
将等离子喷涂制备的陶瓷涂层样品浸入85℃恒温熔融状态十四酸液体中,水浴恒温振荡器中恒温振荡处理30 min,取出样品放入无水乙醇中浸泡并超声清洗10 min,再用蒸馏水清洗材料表面放在空气中干燥待用。十四酸与氧化物相作用机理与图2所示,显示十四酸的羧基在高温下与氧化物产生化学结合,使长碳链外延形成疏水结构。
3. 结果与讨论
3.1. 接触角测量
为了比较十四酸修饰过的陶瓷氧化物涂层疏水性的改变,利用接触测量仪对样品进行表征,发现未经过十四酸修饰的氧化物涂层接触角仅为62˚,而经过十四酸修饰的氧化物陶瓷层接触角值达到了129˚,如图3和图4所示,并经过紫外灯48小时照射老化测试,结果未发现材料表面疏水性衰退现象。
3.2. 盐雾实验
将不同过程制备样品分为三组:a组为仅进行表面喷砂理的Q235钢板,b组为表面喷涂氧化物陶瓷层样品和c经过疏水处理的氧化物陶瓷层。将三组样品均放置于恒温35摄氏度饱合食盐浓雾环境进行30天浓盐雾腐蚀实验。实验结果如图5所示,a喷砂样品表面出现十分严重的腐蚀,铁锈层很厚且出现层结构及部分锈蚀脱落现象;b表面喷涂氧化物陶瓷层对抗盐雾腐蚀有一定作用,表面出现少数几个锈点面积不大;c经过疏水处理的氧化物陶瓷层表面未出现锈迹,与新制备样品表面无明显区别。
3.3. 自然海水浸泡实验
取适量自然海水(海南海口白沙门,位置E:110˚19'N:20˚3')置于500 ml烧杯内,将样品挂于天平底面挂钩片,去除前十秒的海水与表面浸润过程质量剧烈变化过程数据,然后每秒记录三次数据取平均值,共观察记录45,000秒。如图6所示,喷砂处理样品在海水中质量变化十分剧烈,起伏不定,可能由于海水使其表面迅速形成锈层,锈层达到一定厚度在海水作用下溶解再生长过程,使质量变化十分不稳定; 表面喷涂处理的样品表现为质量小幅变化,但是质量总是向增加的方向发展,由于表面吸附海水中的微生物,使表面质量不断增长变化;经疏水处理氧化物涂层质量变化十分微小,显示出最高稳定性,可能即无(或少量)吸附也未发生表面锈蚀。
3.4. 样品表面的微观形貌
如图7电镜照片展示了实验过程不同时期不同测试后样品表面微观形貌。图7(a)为原始喷涂粉的形貌,经激光粒度分析可知微粒尺寸为34微米左右且粒度直径分布窄,与电镜结果一致;该喷涂粉经高温等离子体熔化并喷涂在经新鲜喷砂处理的Q235钢板后形成一层相对致密的氧化物陶瓷层,厚度约为70

Figure 1. Profiles of plasma sprayed Ceramic Coatings formed by AT13 (×800)
图1. 等离子喷涂制备的AT13陶瓷层剖面图(×800)
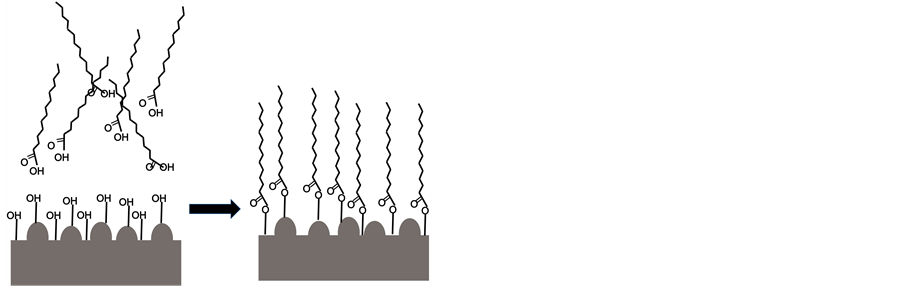
Figure 2. Then interaction between ceramic layer and myristic acid
图2. 氧化物陶瓷层与十四酸相互作用示意

Figure 3. Contact angle of modified ceramic coatings by myristic acid
图3. 经十四酸修饰的氧化物陶瓷涂层的接触角测量

Figure 4. The interactions between modified ceramic coating and water
图4. 经修饰的氧化物陶瓷涂层与水相互作用

Figure 5. The state of the surface of the different processing sample by the 30-days salt spray corrosion test
图5. 经30天盐雾腐蚀实验后不同处理过程样品的表面状态
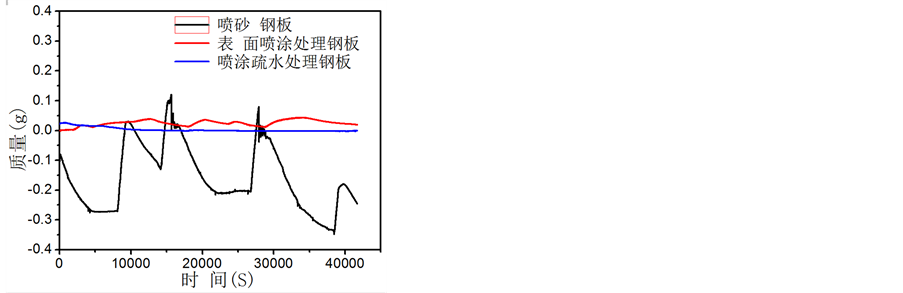
Figure 6. The sample quality changes over time by sea water immersion test
图6. 不同方式制备样品表面海水浸泡测试质量随时间变化

Figure 7. The surface morphology of the sample material used in the spraying process. (a) AT13 powder (b) hydrophobic surface (c) Surface after seawater immersion (d) The surface of the salt fog treatment
图7. 喷涂过程中所用样品材料的表面形貌。(a) AT13喷涂粉;(b) 疏水喷涂表面;(c) 海水浸泡后表面;(d) 盐雾处理后表面

Figure 8. Impedance spectra of different treatment of the surface coating
图8. 不同处理表面的涂层的阻抗谱
微米;该样品浸入到十四酸熔液中涂层进行疏水处理,然后海水浸泡七天表面形貌并没有发生明显变化;经30天盐雾测试后表面出现了大量白色固体沉积物,可能是NaCl晶体或有机物沉积,有待进一步研究确定。
3.5. 电化学阻抗分析
将喷涂样品及疏水处理后的样品置于饱合食盐水中进行电化学阻抗测试,由图8可以看出,喷涂样品及疏水处理后的样品阻抗弧半径均比较大,可以认为电解质未渗入涂层孔隙达到基体,涂层保护基本完好。喷涂疏水样品的阻抗弧半径较单纯喷涂表面的阻抗弧半径更大,可以推断经过疏水处理后喷涂表面的防腐性能更加优秀。
4. 结论
离子喷涂法制备的氧化物陶瓷层在力学及耐久性等方面应用比有机防腐涂料更有优势;本文采用大气热等离子体技术将AT13粉喷涂到Q235钢板表面,并利用十四酸在表面进行修饰得到接触角达129度疏水复合陶瓷涂层。经盐雾实验、海水浸泡实验和电化学等测试均表明,氧化物陶瓷层有一定的防腐效果,氧化物陶瓷层再经疏水处理后防腐性能得到了进一步的提高。
基金项目
国家自然科学基金研究项目(项目编号:51361009);海南大学教育教学研究项目(项目编号:hdjy1225);海南大学青年基金研究项目(项目编号:qnjj1239);海南省自然科学基金(项目编号:20155216)。
文章引用
杨 亮,曹 阳. 等离子喷涂制备疏水涂层的防腐性能研究
Study on Hydrophobic Coating Prepared by Plasma-Spraying Anticorrosion Performance[J]. 材料科学, 2017, 07(02): 189-195. http://dx.doi.org/10.12677/MS.2017.72024
参考文献 (References)
- 1. Diaz, B., Haerkoenen, E., Swiatowska, J., Maurice, V., Seyeux, A., Marcus, P. and Ritala, M. (2011) Low-Tempera- ture Atomic Layer Deposition of Al2O3 Thin Coatings for Corrosion Protection of Steel: Surface and Electrochemical Analysis. Corrosion Science, 53, 2168. https://doi.org/10.1016/j.corsci.2011.02.036
- 2. Zheng, M., Sakairi, M. and Jha, H. (2012) Influence of Desicca-tion Procedures on the Surface Wettability and Corrosion Resistance of Porous Aluminium Anodic Oxide Films. Cor-rosion Science, 55, 332. https://doi.org/10.1016/j.corsci.2011.10.041
- 3. Behzadnasab, M., Mirabedini, S.M., Kabiri, K. and Jamali, S. (2010) Corrosion Performance of Epoxy Coatings Containing Silane Treated ZrO2 Nanoparticles on Mild Steel in 3.5% NaCl Solution. Corrosion Science, 53, 89. https://doi.org/10.1016/j.corsci.2010.09.026
- 4. Abdulagatov, A.I., Yan, Y., Cooper, J.R., Zhang, Y.Z., Gibbs, M., Cavanagh, A.S., Yang, R.G., Lee, Y.C. and George, S.M. (2011) Al2O3and TiO2 Atomic Layer Deposition on Copper for Water Corrosion Resistance. ACS Applied Materials and Interfaces, 3, 4593. https://doi.org/10.1021/am2009579
- 5. Feng, L., Zhang, H., Mao, P., Wang, Y. and Ge, Y. (2011) Super Hy-drophobic Alumina Surface Based on Stearic Acid Modification. Applied Surface Science, 15, 3959-3963.
- 6. Wang, H., Gao, M., Guo, Y., et al. (2016) A Natural Extract of Tobacco Rob as Scale and Corrosion Inhibitor in Arti-ficial Seawater. Desalination, 398, 198-207. https://doi.org/10.1016/j.desal.2016.07.035
- 7. Wang, H., Ning, C., Huang, Y., et al. (2017) Improvement of Abrasion Resistance in Artificial Seawater and Corrosion Resistance in NaCl Solution of 7075 Aluminum Alloy Processed by Laser Shock Peening. Optics and Lasers in Engineering, 90, 179-185. https://doi.org/10.1016/j.optlaseng.2016.10.016
- 8. Sathiyanarayanan, S., Azim, S.S. and Venkatachari, G. (2008) Corrosion Protection Coating Containing Polyaniline Glass Flake Composite for Steel. Electrochimica Acta, 53, 2087-2094. https://doi.org/10.1016/j.electacta.2007.09.015
- 9. Ping, Z.X., He, Y.D., Gu, C.D. and Zhang, T.Y. (2008) Mechanically Assisted Electroplating of Ni-P Coatings on Carbon Steel. Surface and Coatings Technology, 202, 6023. https://doi.org/10.1016/j.surfcoat.2008.06.183
- 10. Mu, S.L., Li, N., Li, D.Y. and Zou, Z.L. (2009) Inves-tigation of a Transparent Chromate(III) Passive Film on Electroless Ni-P Coating by XPS and Electrochemical Methods. Electrochimica Acta, 54, 6718-6724. https://doi.org/10.1016/j.electacta.2009.06.076
- 11. Tian, J.T., Liu, X.Z., Wang, J.F., Wang, X. and Yin, Y.S. (2010) Electrochemical Anticorrosion Behaviors of the Electroless Deposited Ni–P and Ni–P–PTFE Coatings in Steri-lized and Unsterilized Seawater. Materials Chemistry and Physics, 124, 751-759. https://doi.org/10.1016/j.matchemphys.2010.07.053
- 12. Doeslu, S.T., Mert, B.D. and Yazici, B. (2013) Poly-indole Top Coat on TiO2 Sol—Gel Films for Corrosion Protection of Steel. Corrosion Science, 66, 51-58. https://doi.org/10.1016/j.corsci.2012.08.067
- 13. Fujita, R., Sakairi, M., Kikuchi, T. and Nagata, S. (2011) Cor-rosion Resistant TiO2 Film Formed on Magnesium by Liquid Phase Deposition Treatment. Electrochimica Acta, 56, 7180-7188. https://doi.org/10.1016/j.electacta.2011.03.146
- 14. Yabuki, A. and Sakai, M. (2011) Self-Healing Coatings of Inorganic Particles Using a pH Sensitive Organic Agent. Corrosion Science, 53, 829-833. https://doi.org/10.1016/j.corsci.2010.11.021
- 15. Yu, D.Y., Wang, J.F., Tian, J.T., Xu, X.M., Dai, J.H. and Wang, X. (2013) Preparation and Characterization of TiO2/ZnO Composite Coating on Carbon Steel Surface and Its Anticor-rosive Behavior in Seawater. Composites Part B: Engineering, 46, 135-144. https://doi.org/10.1016/j.compositesb.2012.10.036
- 16. Boinovich, L.B., Gnedenkov, S.V., Alpysbaeva, D.A., Egorkin, V.S., Emelyanenko, A.M., Sinebryukhov, S.L. and Zaretskaya, A.K. (2012) Corrosion Resistance of Compo-site Coatings on Low-Carbon Steel Containing Hydrophobic and Super Hydrophobic Layers in Combination with Oxide Sub-Layers. Corrosion Science, 55, 238. https://doi.org/10.1016/j.corsci.2011.10.023
- 17. Wang, P., Zhang, D., Qiu, R. and Hou, B.R. (2012) Su-per-Hydrophobic Film Prepared on Zinc as Corrosion Barrier. Corrosion Science, 53, 2080. https://doi.org/10.1016/j.corsci.2011.02.025
- 18. Li, M., Yang, Y.Q., Liu, L., Hu, J.M. and Zhang, J.Q. (2010) Electro-Assisted Preparation of Dodecyltrimethoxysilane /TiO2 Composite Film for Corrosion Protection of AA2024-T3 (Aluminum Alloy). Electrochimica Acta, 55, 3008. https://doi.org/10.1016/j.electacta.2009.12.081
- 19. Yuan, S.J., Pehkonen, S.O., Liang, B., Ting, Y.P., Neoh, K.G. and Kang, E.T. (2012) Super Hydrophobic Fluoropolymer Modified Copper Surface via Surface Graft Polymerisation for Corrosion Protection. Corrosion Science, 53, 2738. https://doi.org/10.1016/j.corsci.2011.05.008
- 20. Chen, N., Liu, M.Y. and Zhou, W.D. (2012) Fouling and Corrosion Properties of SiO2 Coatings on Copper in Geothermal Water. Industrial and Engineering Chemistry Research, 51, 6001. https://doi.org/10.1021/ie202091b
*通讯作者。
