Journal of Advances in Physical Chemistry
Vol.06 No.02(2017), Article ID:20625,13
pages
10.12677/JAPC.2017.62011
Research Progress on Improving the Photocatalysis of Graphite-C3N4 via O, S and P Doping
Ran You, Lu Chen, Yongping Zhang
Faculty of Materials and Energy, Southwest University, Chongqing

Received: May 4th, 2017; accepted: May 21st, 2017; published: May 24th, 2017

ABSTRACT
Graphitic arbon nitride (g-C3N4) has attracted extensive attention in the field of photocatalysis because of its unique atomic and electronic structures. Some intrinsic characteristics, such as small specific surface area and rapid recombination of the photogenerated electron-hole pair, restrict its application. It is a valid pathway to improve the photocatalytic performance of g-C3N4 by changing its physical and chemical properties via atomic doping. In this paper, we overview the ways to optimize the photocatalysis of g-C3N4, and focus on the techniques and effect of atomic doping. Finally, the research perspective on the g-C3N4 is discussed.
Keywords:Template, Graphitic-C3N4, Atomic Doping, Photocatalytic Performance
原子掺杂改善石墨相氮化碳光催化性能的 研究进展
游然,陈露,张永平
西南大学材料与能源学部,重庆
收稿日期:2017年5月4日;录用日期:2017年5月21日;发布日期:2017年5月24日

摘 要
石墨相氮化碳(g-C3N4)独特的结构使其在光催化性能上具有一定的优越性,使其研究受到了广泛的关注。但g-C3N4较低的比表面积以及较高的光生载流子复合率限制了其应用,为了进一步改善g-C3N4的性能,通过原子和分子掺杂改变它的电子结构来改变其物理和化学性质是一个有效途径。本文就如何优化g-C3N4的光催化性能做了简单的概述,重点介绍了非金属原子O, S, P掺杂对g-C3N4性能的影响,并展望了未来g-C3N4研究的动向。
关键词 :模板,g-C3N4,原子掺杂,光催化性能

Copyright © 2017 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


1. 引言
人类社会的加速发展使得能源短缺和环境污染问题越来越严重。如何利用光催化剂将太阳能转换为可直接利用的能源,或者使污染物降解是当今研究的热点之一。人们关注的一些光催化剂TiO2、ZnO、BiWO6等半导体材料在结构上或反应过程中存在一定缺陷,如,CB(导带)位置相对较低,使得其光生电子的还原能力较弱 [1] [2] ;或能带间隙较大,使其对可见光的吸收能力有限 [3] ;或会随着反应过程中贵金属的沉积表现出不稳定状态 [4] [5] [6] 。石墨型C3N4(g-C3N4)作为一种具有可见光响应的非金属半导体材料 [7] ,具有独特的材料结构和电子结构,在性能上具有一定的优越性。
g-C3N4是以七嗪环(C6N7)为基本单元聚合而成的π共轭体系 [8] [9] 形成的二维平面层状结构,面内排列距离为0.73 nm;层与层之间通过范德瓦耳斯力结合,层间距离为0.32 nm [10] ,如图1所示。其结构类似于石墨烯,但是不同于零能带间隙的石墨烯 [11] ,g-C3N4禁带宽度为2.7 eV [12] ,可以吸收太阳光谱中波长小于475 nm的蓝紫光。在参比电极为标准氢电极,电解液为中性溶液时测得g-C3N4的CB位置大约在-1.23 V,比光催化降解CO2生成碳氢化合物的位置更负(CH4约-0.24 V、CH3OH约-0.38 V) [13] [14] ,g-C3N4的VB(价带)位置大约在1.47 V,比分解H2O制O2的位置更正。g-C3N4特殊的结构使其具有极好的热稳定性(在空气中,600℃依然稳定)和化学稳定性(在酸、碱中) [15] 。综上所述,g-C3N4适用于各种化学反应 [16] ,例如,光催化生产H2O2 [17] 、降解CO2 [18] [19] 、光分解H2O制H2 [20] 和O2 [21] 、降解有机染料 [22] 等。
g-C3N4由地球上大量存在的元素C、N组成,因此,g-C3N4可以由富C和富N的前驱体合成 [23] ,例如,三聚氰胺 [24] [25] ,硫脲 [18] ,尿素 [26] [27] 等。这些前驱体价格便宜且储量丰富 [28] ,适宜于大规模的生产。但是,相对于g-C3N4的诸多优点,g-C3N4还有一些不足之处,如,较低的载流子迁移能力;有限的可见光吸收范围;较高的光生电子-空穴复合率;较低的比表面积 [29] 。因此人们用了很多的方法去改良g-C3N4,如,原子 [30] - [60] 或分子 [61] [62] [63] 掺杂、剥离成2D纳米薄片 [64] [65] [66] 、改变g-C3N4的形貌 [67] - [74] 、与半导体材料形成异质结 [75] [76] [77] [78] [79] 、与金属等其他材料耦合 [80] [81] [82] [83] 。
通过非金属(O [30] - [35] 、S [36] - [41] 、P [42] - [49] 、B [50] [51] [52] [53] 、I [54] 、C [55] [56] )或金属原子(Ag [57] 、Co [58] 、Fe [59] [60] )的掺杂可以改变g-C3N4的电子结构和能带间隙;扩大对可见光的吸收范围和吸收强度;提高载流子的传输效率;增大比表面积以增加反应的活性位点。原子掺杂是一种应用广泛且可以有效改良g-C3N4性能的方法。其中O、S、P原子的掺杂更是应用广泛,本文主要就O、S、P这三种非金属原子的掺杂方法、相应的方法原理及其性能做了评述。
 (a)
(a) (b)
(b)
Figure 1. (a) planar and (b) interlayer structural illustration of g-C3N4 (C, N indicated by gray, blue spheres, respectively). A heptazine unit (C6N7) is highlighted in yellow [10]
图1. g-C3N4平面(a)及层间(b)结构示意图(C, N原子分别用灰色,蓝色圆球表示), 七嗪环(C6N7) 单元用黄色标出 [10]
2. O原子的掺杂
众所周知,g-C3N4和石墨烯拥有相似的结构以及化学性质,而石墨烯的光电性质与石墨烯的含O水平有极其紧密的联系 [84] 。因此,人们猜想g-C3N4的性能是否也与其含O量有关 [35] 。O原子的电负性是3.44 eV,N原子的电负性是3.04 eV。O原子相对较高的电负性有利于限制电子的移动 [34] 。因此,N-O、C-O、C=O这些含O官能团可作为吸电子集团,在光催化反应中促进电子-空穴的分离这方面扮演着很重要的角色。
2.1. 水热法
水热法是形成含O官能团,进行O掺杂的常用手段。常以H2O2作为反应溶剂 [33] [34] [35] 。Li等人 [33] 以H2O2作为反应溶剂,在反应釜中水热合成O-g-C3N4(O原子掺杂的g-C3N4),实验测得其降解亚甲基蓝的速率是体相g-C3N4的4倍,制H2速率是其2.5倍。
Ming等人 [35] 将制得的g-C3N4在180℃的条件下,分别水热不同时间,发现在水热12 h时,g-C3N4的含O量达到最大,继续水热,含O量基本不变。且水热12 h所得g-C3N4的光载流子响应可以达到体相g-C3N4的10倍,光催化降解AO7 (酸性橙7)的速率可以达到其7倍。
2.2. 超分子自组装
水超分子自组装是利用分子间氢键的方向性合成在结构上高度有序的分子聚集体的合成过程。其高度有序的结构可以减少缺陷的产生,而缺陷(g-C3N4在脱氨基过程中产生的NH/NH2组织)通常会成为光生电子-空穴的复合中心,因而超分子合成有利于光生电子-空穴的分离,从而促进g-C3N4光电性能的提高。
Huang等人 [31] 用H2O2前处理三聚氰胺,使其在氢键作用下形成凝胶状物质,该过程为三聚氰胺的超分子自组装过程。然后,将所得凝胶状物质离心,干燥,研磨。再将所得的粉末状物质以5℃/min的速率加热到550℃并保温2 h,得到多孔且掺O的g-C3N4。如图2所示为前处理成过程。这种前处理方法简单,但是可以直接合成既有一定形貌特点又掺杂O原子的g-C3N4,为原子掺杂与形貌改良共进的多重优化方式提供了参考价值,这种方法有效改善了g-C3N4的光电性能。
2.3. 芬顿反应
水H2O2与二价铁离子Fe2+的混合溶液为芬顿试剂,具有很强的氧化性,可以将很多的有机化合物氧化为无机态。Guo等人 [30] 将合成的体相g-C3N4用酸刻蚀剥离形成g-C3N4薄片,然后,再用芬顿试剂处理,芬顿反应如图3所示。芬顿试剂的强氧化性可以使原先剥离出来的g-C3N4薄片上的缺陷位点氧化形成孔隙,还可以使O原子掺入到g-C3N4薄片的边缘,形成含有多孔结构且边缘掺O的g-C3N4薄片。
水在对g-C3N4进行O原子掺杂的过程中人们发现,O原子的掺入确实可以促进电子-空穴的分离,减小g-C3N4的能带间隙,提高g-C3N4对可见光的吸光范围。
3. S原子的掺杂
阴离子掺杂是一种可以有效提高g-C3N4可见光吸收范围的方法 [38] [39] 。另外,掺杂具有较高元素周期序数的元素也可以降低g-C3N4的能带间隙,从而扩大对可见光的吸收范围 [41] 。S元素既具有较高的元素周期序数,同时又可作为阴离子掺杂改良g-C3N4,因此受到了人们的关注。

Figure 2. Illustration of fabrication of g-C3N4 with porous network and O-doping by hydrogen bond-induced supramolecular assembly (C, N and substitutional O are indicated by gray, blue and red spheres, respectively [31]
图2. (a)超分子自组装合成O原子掺杂且多孔g-C3N4的示意图(灰、蓝、红色的小球分别代表C、N、O原子) [31]

Figure 3. Schematic illustration of the O-g-C3N4 thin sheets formed via photo-Fenton reaction [30]
图3. 芬顿反应合成O-g-C3N4薄片示意图 [30]
根据S源的性质,我们把掺S的方法分为两大类,1、以H2S气体为S源,在S源的气体氛围下合成S-g-C3N4(S原子掺杂的g-C3N4)。2、分别以TU(硫脲)、TCA(三聚硫氰酸)为S源自合成S-g-C3N4。
3.1. S源作为气体氛围
比结构中替代原子具有更低电负性的原子的匀质掺杂可以增加VB的宽度。VB的宽度影响并控制空穴的移动。VB的宽度越宽,空穴的移动性越强,其氧化作用越强。但是,原子的匀质掺杂因为其有限的溶解度而很难实现。
Liu等人 [39] 以双氰胺为前驱体,合成g-C3N4,研磨后置于H2S气体(纯度,99.99%,通气速率,12 ml/min)的氛围下,升温到450℃再保温1 h,合成S-g-C3N4。通过长时间的Ar+刻蚀发现S的2p峰并没有出现变化,证明在H2S气体的氛围下,实现了对S的匀质掺杂。S掺杂的g-C3N4在λ > 400 nm时仍然可以完全氧化苯酚,但是这个氧化过程对于未掺杂的g-C3N4来说,在λ > 300 nm时已然不可能发生。说明S的匀质掺杂确实扩大了对可见光的响应范围,增强了氧化性。
3.2. 自合成
不同于以S源做为气体氛围时需要先制备Bulk-g-C3N4(体相g-C3N4),以TCA [37] [41] 和TU [36] [38] [40] 做为前驱体可以直接合成S-g-C3N4。
以富C、N材料(如,氨腈、双氰胺、三聚氰胺)作为前驱体时,由于动力学的原因,聚合并不完全 [10] 。而由于不完全聚合产生的缺陷(如,NH/NH2组织)可作为光生电子-空穴的复合中心,降低载流子的浓度,从而减小g-C3N4的活性 [85] 。
Zhang等人 [41] 以TCA为前驱体,自合成了S-g-C3N4。合成过程中-SH代替原先的氨基组织,成为新的游离基,促进了g-C3N4的聚合。实验证明新合成的S-g-C3N4的制H2能力是传统未掺杂g-C3N4的14倍。通常,分解H2O制O2很难通过单一的催化剂催化实现,但是,该方法合成的g-C3N4制O2能力是传统g-C3N4的5倍,因此,其可以在没有酸辅助的作用下催化H2O制O2,且能达到可观的速率。
Hong等人 [38] 以TU为前驱体,自合成了S-g-C3N4,其合成机理如图4。Hong等人认为TU在热聚合的过程中有中间产物(氨腈、三聚氰胺、三聚氰酸等)生成,中间产物TCA的-SH和中间产物氨腈或三聚氰胺的-NH作用,促进S原子进入g-C3N4的结构中。经试验测试得到,用硫脲自合成得到的S-g-C3N4的制H2能力可以达到g-C3N4的10倍。

Figure 4. Proposed formation mechanism of sulfur-doped g-C3N4 from condensation of thiourea [38]
图4. 硫脲合成S掺杂g-C3N4的过程示意图 [38]
4. P原子掺杂
P原子的掺入可以抑制晶体的生长,扩大g-C3N4的比表面积,为催化反应的进行提供更多的反应活性位点 [86] ;还可以改良g-C3N4的电子结构 [45] [49] 。如图5所示,当P原子取代C位后,P原子3/5的价电子与N形成P-N共价键,剩余的孤电子对参与到三嗪环的π共轭离域体系,剩下P+成为路易斯酸位点,促进光生电子和空穴的分离;另外,g-C3N4上的路易斯碱位点(-NH2)和路易斯酸位点(P+)构成的酸碱双功能团有助于实现对环氧衍生物的CO2环加成 [44] 。现在我们就P的掺杂方法进行了总结。
4.1. 简单混合热聚合
简单混合热聚合是指将P源和生成g-C3N4的前驱体进行简单混合,再加热保温处理的过程。其方法简单,温和,但合成的P-g-C3N4(P原子掺杂的g-C3N4)的活性受到P源的影响。
离子液体通常指熔化温度小于100℃的有机盐液体,其全部由离子组成。离子液体具有较高的化学稳定性和热稳定性,因而被广泛应用在很多的领域 [87] 。
Zhang和他的团队 [48] 以离子液体BmimBF6和双氰胺作为前驱体,合成了P-g-C3N4。在反应过程中,BmimBF6因具有较高的热稳定性,不会在双氰胺热聚合之前发生分解,但随着温度的继续升高,PF6-与氨基集团作用,使P原子成功掺入到C-N的结构。之后,Zhang、Chen等人 [47] 在以相同的P源和前驱体合成的P-g-C3N4中发现了多孔结构,其认为BmimBF6不仅作为P源参与反应而且在聚合过程中充当了软模板的角色,产生了碳氢化合物和NH4F等可以分解产生气体的物质。
用离子溶液作为P源方法简单,但其价格昂贵,且掺P量较少,对g-C3N4性能的改善有限。所以Hu等人 [43] 将P源改为(NH4)2HPO4,制得P-g-C3N4。同时,作为对比,以相同质量比的离子液体BmimBF6作为P源,用相同方法制得P-g-C3N4,记为IL- P-g-C3N4,研究它们制氢能力。发现P-g-C3N4的制氢能力是IL-P-g-C3N4的1.8倍。
如图6(a)、图6(b)所示,是分别以BmimBF6、(NH4)2HPO4为P源的P原子的两种可能的掺杂位置。不同的P源会影响P原子在g-C3N4结构中的掺杂位置以及掺杂量,从而导致不同的催化能力 [43] 。所以人们致力于寻求合适的P源,来获得性能理想的P-g-C3N4。
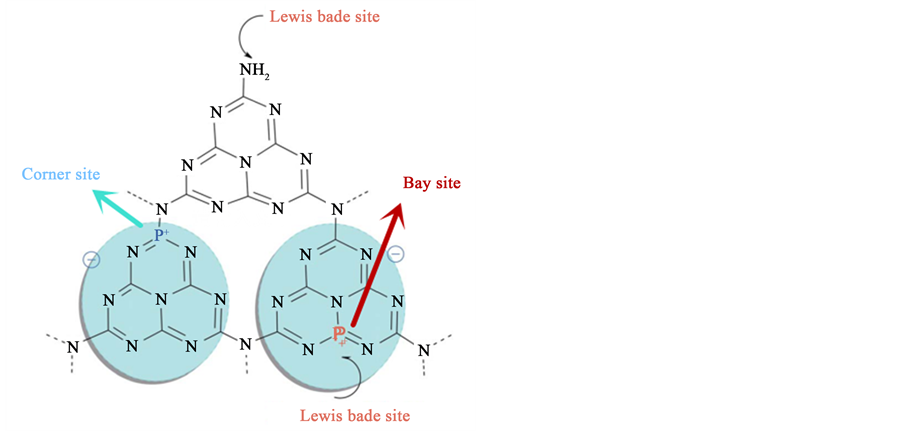
Figure 5. The electronic structure and the possible doping sites of P-g-C3N4. [49]
图5. P-g-C3N4的电子结构及可能的P原子的掺杂位置 [49]
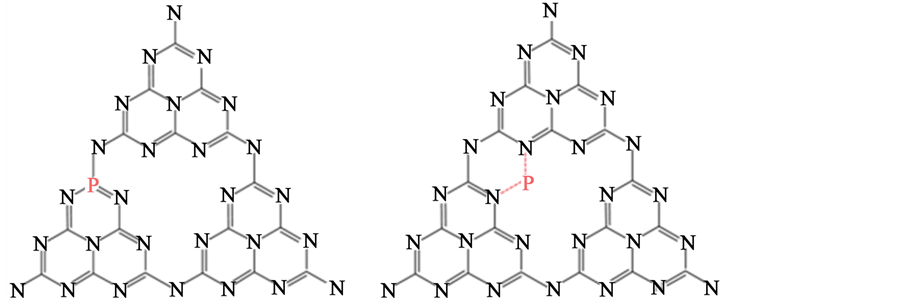 (a) (b)
(a) (b)
Figure 6. The possible doping sites of P atoms in g-C3N4 by using (a) BmimBF6 and (b)(NH4)2HPO4 as precursor [43]
图6. (a) BmimBF6为前驱体时P原子可能掺杂的位置;(b) (NH4)2HPO4为前驱体时P原子可能掺杂的位置 [43]
Zhou等人 [49] 和Lan [44] 等人将HCCP(六氯环三磷腈)作为P源,HCCP的价格要优于BmimBF6,同时,HCCP拥有类三嗪的P-N环结构与g-C3N4的结构非常吻合,且P-Cl键非常活泼,可以在聚合过程中与-NH2发生反应,促进脱氨基过程的进行,降低反应温度以及引入P原子。图5所示为以HCCP作为P源时,P原子的掺杂位置示意图。实验显示以HCCP作为P源合成的P-g-C3N4降解有机染料罗丹明的速率是一般g-C3N4的3倍,制氢速率是一般g-C3N4的2.9倍 [49] ,另外,在对环氧衍生物加成CO2也有出色的催化效果 [44] 。
4.2. 酸-碱作用
P源和生成g-C3N4的前驱体之间不再只是简单的混合,它们之间会因为酸碱作用,紧密地交联在一起。Ran等人 [46] 用三聚氰胺和AEP(2-氨乙基磷酸)合成了多孔的P-g-C3N4。合成示意图如图7所示。三聚氰胺与AEP之间的酸碱作用和范德瓦耳斯力促进了均匀介孔结构的产生,使P-g-C3N4具有较大的比表面积,有利于增加其催化能力。
Ma等人 [45] 用碳纤维纸、三聚氰胺、乙二磷酸为先驱体合成了花状结构的P-g-C3N4,其合成示意图如图8所示。碳纤维纸、三聚氰胺、乙二磷酸之间的酸碱作用,促进前驱体吸附在碳纤维纸上,有利于P-g-C3N4花状纳米结构的生长及P-g-C3N4与碳纤维纸的耦合。
总之,P源和生成g-C3N4的前驱体之间的酸碱作用力在促进P原子引入结构的同时,有助于其他类似介孔的匀质结构及可控形貌的生成,从而在掺杂与形貌的多作用力下增强P-g-C3N4的催化能力。
4.3. 超分子自组装
与O原子掺杂时所用的方法类似,P原子的掺杂也可以通过超分子自组装来实现,所用溶剂除了在提供掺杂原子上有所区别外,也会影响g-C3N4结构的变化,不同溶剂会合成不同形貌的g-C3N4 [88] 。
Guo和他的团队 [42] 以三聚氰胺和H3PO4作为反应的原材料合成了具有六方棒状的P-g-C3N4。其中H3PO4水溶液的作用有三,1、H3PO4提供了一定的酸性环境,辅助水解三聚氰胺形成三聚氰酸。2、H3PO4作为P源合成P-g-C3N4。3、作为超分子自组装的溶剂。有文献研究表明,作为g-C3N4前驱体的三聚氰胺可以和三嗪衍生物,如,三聚氰酸,通过氢键形成具有特定形貌的超分子结构 [88] [89] [90] 。在Guo的实验中,三聚氰胺和经H3PO4辅助水解得到的三聚氰酸自组装合成具有六方棒状的超分子前驱体——MCA,H3PO4分子吸附在MCA的表面,再经过N2作为保护气体的热聚合反应后,P原子进入g-C3N4的结构中。具体的合成过程可参见图9。

Figure 7. Synthesis procedure of porous P-g-C3N4 using acid-base interaction [46]
图7. 通过酸-碱作用合成多孔P-g-C3N4示意图 [46]

Figure 8. Fabrication of P-g-C3N4 nanostructures directly grown on CFP usingacid-base interaction. [45]
图8. 通过酸碱作用在CFP上合成纳米结构的P-g-C3N4。 [45]

Figure 9. The formation process of phosphorus-doped tubular carbon nitride by supramolecular assembly [42]
图9. 通过合成过超分子自组装原理合成P-g-C3N4的管状结构 [42]
该研究通过对前驱体的处理,在引入掺杂原子的同时,自组装改变了g-C3N4的形貌,是实现多重优化的有利手段。
5. 双原子掺杂
单一的原子掺杂已经对g-C3N4改性起到了有效作用,双原子掺杂可以协同影响g-C3N4的性能,使其具有更好的光催化效果。
You等人 [91] 以三聚硫氰酸为S源,以过氧化氢为O源合成了S,O原子双掺杂的g-C3N4,研究表明,单掺S原子的g-C3N4光催化性能是纯的体相g-C3N4的1.5倍,但双原子掺杂后其光催化性能为纯的体相g-C3N4的6倍。显然,双原子掺杂起到了很好的协同作用,有效改善了g-C3N4的光催化性能。
6. 结论与展望
g-C3N4是一种类似于石墨烯的层状非金属半导体材料,具有独特的电子结构和极好的热稳定性、化学稳定性和良好的光催化性能,因而受到了科学家的关注。但是g-C3N4的性能却受制于其有限可见光的吸收范围、较小的比表面积及较快的光电子-空穴复合率。改良g-C3N4光催化性能有多种手段,原子掺杂是其中应用最为广泛的,同时,也是最有效的手段之一。单一的原子掺杂就可以实现对g-C3N4性能的有效改善,而利用双原子掺杂或者利用超分子自组装,分子间酸-碱作用力这样的方法引入掺杂原子,同时还可以实现掺杂原子协同作用或掺杂与形貌结构共进的多重优化,有利于促进g-C3N4性能的改善,可能成为今后g-C3N4改性研究的发展方向。
资助信息
国家自然科学基金(批准号,21173170);中央高校基本科研业务费资助项目(XDJK2016D002)。
文章引用
游 然,陈 露,张永平. 原子掺杂改善石墨相氮化碳光催化性能的研究进展
Research Progress on Improving the Photocatalysis of Graphite-C3N4 via O, S and P Doping[J]. 物理化学进展, 2017, 06(02): 84-96. http://dx.doi.org/10.12677/JAPC.2017.62011
参考文献 (References)
- 1. Chen, X., Zhou, Y., Liu, Q., et al. (2012) Ultrathin Single-Crystal WO3 Nanosheets by Two-Dimensional Oriented Attachment toward Enhanced Photocatalystic Reduction of CO2 into Hydrocarbon Fuels under Visible Light. ACS Applied Materials & Interfaces, 4, 3372-3377. https://doi.org/10.1021/am300661s
- 2. Zhou, Y., Tian, Z., Zhao, Z., et al. (2011) High-Yield Synthesis of Ultrathin and Uniform Bi2WO6 Square Nanoplates Benefitting from Photocatalytic Reduction of CO2 Intorenewable Hydrocarbon Fuel under Visible Light. ACS Applied Materials & Interfaces, 3, 3594-3601. https://doi.org/10.1021/am2008147
- 3. Xi, G., Ouyang, S. and Ye, J. (2011) General Synthesis of Hybrid TiO2 Mesoporous “French Fries” toward Improved Photocatalytic Conversion of CO2 into Hydrocarbon Fuel, a Case of TiO2/ZnO. Chemistry, 17, 9057-9061.
- 4. He, Y., Zhang, L., Teng, B., et al. (2015) New Application of Z-Scheme Ag3PO4/G-C3N4 Composite in Converting CO2 to Fuel. Environmental Science & Technology, 49, 649-656. https://doi.org/10.1021/es5046309
- 5. Hu, J.S., Ren, L.L., Guo, Y.G., et al. (2005) Mass Production and High Photocatalytic Activity of ZNS Nanoporous Nanoparticles. Angewandte Chemie International Edition in English, 44, 1269-1273.
- 6. Li, Q., Guo, B., Yu, J., et al. (2011) Highly Efficient Visible-Light-Driven Photocatalytic Hydrogen Production of CdS-Cluster-Decorated Graphene Nanosheets. Journal of the American Chemical Society, 133, 10878-10884. https://doi.org/10.1021/ja2025454
- 7. Shalom, M., Gimenez, S., Schipper, F., et al. (2014) Controlled Carbon Nitride Growth on Surfaces for Hydrogen Evolution Electrodes. Angewandte Chemie International Edition in English, 53, 3654-3658. https://doi.org/10.1002/anie.201309415
- 8. Chen, L., Huang, D., Ren, S., et al. (2013) Preparation of Graphite-Like Carbon Nitride Nanoflake Film with Strong Fluorescent and Electrochemiluminescent Activity. Nanoscale, 5, 225-230. https://doi.org/10.1039/C2NR32248J
- 9. Gong, Y., Li, M., Li, H., et al. (2015) Graphitic Carbon Nitride Polymers, Promising Catalysts or Catalyst Supports for Heterogeneous Oxidation and Hydrogenation. Green Chemistry, 17, 715-736. https://doi.org/10.1039/C4GC01847H
- 10. Bojdys, M.J., Muller, J.O., Antonietti, M., et al. (2008) Ionothermal Synthesis of Crystalline, Condensed, Graphitic Carbon Nitride. European Journal of Inorganic Chemistry, 14, 8177-8182. https://doi.org/10.1002/chem.200800190
- 11. Castro, E.V., Novoselov, K.S., Morozov, S.V., et al. (2007) Biased Bilayer Graphene, Semiconductor with a Gap Tunable by the Electric Field Effect. European Journal of Inorganic Chemistry, 99, Article ID: 216802. https://doi.org/10.1103/physrevlett.99.216802
- 12. Wang, X., Maeda, K., Thomas, A., et al. (2009) A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nature Materials, 8, 76-80. https://doi.org/10.1038/nmat2317
- 13. Wang, C.J., Thompson, R.L, Baltrus, J., et al. (2010) Visible Light Photoreduction of CO2 Using CdSe/Pt/TiO2 Heterostructured Catalysts. The Journal of Physical Chemistry Letters, 1, 48-53. https://doi.org/10.1021/jz9000032
- 14. Marszewski, M., Cao, S., Yu, J., et al. (2015) Semiconductor-Based Photocatalytic CO2 Conversion. Materials Horizons, 2, 261-278. https://doi.org/10.1039/C4MH00176A
- 15. Zhu, J., Xiao, P., Li, H., et al. (2014) Graphitic Carbon Nitride, Synthesis, Properties and Applications in Catalysis. ACS Applied Materials & Interfaces, 6, 16449-16465.
- 16. Thomas, A., Fischer, A., Goettmann, F., et al. (2008) Graphitic Carbon Nitride Materials, Variation of Structure and Morphology and Their Use as Metal-Free Catalysts. Journal of Materials Chemistry, 18, 4893-4908. https://doi.org/10.1039/b800274f
- 17. Li, S., Dong, G., Hailili, R., et al. (2016) Effective Photocatalytic H2O2 Production under Visible Light Irradiation at G-C3N4 Modulated by Carbon Vacancies. Applied Catalysis B, 190, 26-35. https://doi.org/10.1016/j.apcatb.2016.03.004
- 18. Wang, H., Sun, Z., Li, Q., et al. (2016) Surprisingly Advanced CO2 Photocatalytic Conversion over Thiourea Derived G-C3N4 with Water Vapor while Introducing 200-420nm UV Light. Journal of CO2 Utilization, 14, 143-151. https://doi.org/10.1016/j.jcou.2016.04.006
- 19. Li, M., Zhang, L., Fan, X., et al. (2015) Highly Selective CO2 Photoreduction to CO over G-C3N4/Bi2WO6 Composites under Visible Light. Journal of Materials Chemistry, 3, 5189-5196.
- 20. Martín-Ramos, P., Martín-Gil, J., Dante, R.C., et al. (2015) A Simple Approach to Synthesize G-C3N4 with High Visible Light Photoactivity for Hydrogen Production. International Journal of Hydrogen Energy, 40, 7273-7281.
- 21. Li, Z., Kong, C. and Lu, G. (2016) Visible Photocatalytic Water Splitting and Photocatalytic Two-Electron Oxygen Formation over Cu-and Fe-Doped G-C3N4. The Journal of Physical Chemistry C, 120, 56-63. https://doi.org/10.1021/acs.jpcc.5b09469
- 22. Bhowmik, T., Kundu, M.K. and Barman, S. (2015) Ultra Small Gold Nanoparticles-Graphitic Carbon Nitride Composite, an Efficient Catalyst for Ultrafast Reduction of 4-Nitrophenol and Removal of Organic Dyes from Water. RSC Advances, 5, 38760-38773.
- 23. Cao, S., Low, J., Yu, J., et al. (2015) Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Advanced Materials, 27, 2150-2176.
- 24. Yan, S.C., Li, Z.S. and Zou, Z.G. (2009) Photodegradation Performance of G-C3N4 Fabricated by Directly Heating Melamine. Langmuir, 25, 10397-10401. https://doi.org/10.1021/la900923z
- 25. Komatsu, T. (2001) Attempted Chemical Synthesis of Graphite-Like Carbon Nitride. Journal of Materials Chemistry, 11, 799-801.
- 26. Martin, D.J., Qiu, K.P., Shevlin, S.A., et al. (2014) Highly Efficient Photocatalytic H2 Evolution from Water Using Visible Light and Structure-Controlled Graphitic Carbon Nitride. Angewandte Chemie International Edition, 53, 9240-9245. https://doi.org/10.1002/anie.201403375
- 27. Zhang, Y., Liu, J., Wu, G., et al. (2012) Porous Graphitic Carbon Nitride Synthesized via Direct Polymerization of Urea for Efficient Sunlight-Driven Photocatalytic Hydrogen Production. Nanoscale, 4, 5300-5303.
- 28. Fina, F., Menard, H. and Irvine, J.T. (2015) The Effect of Pt NPs Crystallinity and Distribution on the Photocatalytic Activity of Pt-G-C3N4. Physical Chemistry Chemical Physics, 17, 13929-13936. https://doi.org/10.1039/C5CP00560D
- 29. Shi, H., Chen, G., Zhang, C., et al. (2014) Polymeric G-C3N4 Coupled with NaNbO3 Nanowires toward Enhanced Photocatalytic Reduction of CO2 into Renewable Fuel. ACS Catalysis, 4, 3637-3643.
- 30. Guo, S., Zhu, Y., Yan, Y., et al. (2016) Holey Structured Graphitic Carbon Nitride Thin Sheets with Edge Oxygen Doping via Photo-Fenton Reaction with Enhanced Photocatalytic Activity. Applied Catalysis B: Environmental, 185, 315-321. https://doi.org/10.1016/j.apcatb.2015.11.030
- 31. Huang, Z.F., Song, J., Pan, L., et al. (2015) Carbon Nitride with Simultaneous Porous Network and O-Doping for Efficient Solar-Energy-Driven Hydrogen Evolution. Nano Energy, 12, 646-656.
- 32. Kharlamov, A., Bondarenko, M. and Kharlamova, G. (2016) Method for the Synthesis of Water-Soluble Oxide of Graphite-Like Carbon Nitride. Diamond and Related Materials, 61, 46-55. https://doi.org/10.1016/j.diamond.2015.11.006
- 33. Li, J., Shen, B., Hong, Z., et al. (2012) A Facile Approach to Synthesize Novel Oxygen-Doped G-C3n4 with Superior Visible-Light Photoreactivity. Chemical communications, 48, 12017-12019.
- 34. Liu, S., Li, D., Sun, H., et al. (2016) Oxygen Functional Groups in Graphitic Carbon Nitride for Enhanced Photocatalysis. Journal of Colloid and Interface Science, 468, 176-182.
- 35. Ming, L., Yue, H., Xu, L., et al. (2014) Hydrothermal Synthesis of Oxidized G-c3N4 and Its Regulation of Photocatalytic Activity. Journal of Materials Chemistry A, 2, 19145-19149.
- 36. Cao, L., Wang, R. and Wang, D. (2015) Synthesis and Characterization of Sulfur Self-Doped G-C3N4 with Efficient Visible-Light Photocatalytic Activity. Materials Letters, 149, 50-53. https://doi.org/10.1016/j.matlet.2015.02.119
- 37. Chen, J., Hong, Z., Chen, Y., et al. (2015) One-Step Synthesis of Sulfur-Doped and Nitrogen-Deficient G-C3n4 Photocatalyst for Enhanced Hydrogen Evolution under Visible Light. Materials Letters, 145, 129-132.
- 38. Hong, J., Xia, X., Wang, Y., et al. (2012) Mesoporous Carbon Nitride with In Situ Sulfur Doping for Enhanced Photocatalytic Hydrogen Evolution from Water under Visible Light. Journal of Materials Chemistry, 22, 15006-15012. https://doi.org/10.1039/c2jm32053c
- 39. Liu, G., Niu, P., Sun, C.H., et al. (2010) Unique Electronic Structure Induced High Photoreactivity of Sulfur-Doped Graphitic C3N4. Journal of the American Chemical Society, 132, 11642-11648. https://doi.org/10.1021/ja103798k
- 40. Wang, K., Li, Q., Liu, B., et al. (2015) Sulfur-Doped G-C3n4 with Enhanced Photocatalytic Co2-Reduction Performance. Applied Catalysis B: Environmental, 176, 44-52.
- 41. Zhang, J., Sun, J., Maeda, K., et al. (2011) Sulfur-Mediated Synthesis of Carbon Nitride, Band-Gap Engineering and Improved Functions for Photocatalysis. Energy & Environmental Science, 4, 675-678. https://doi.org/10.1039/C0EE00418A
- 42. Guo, S., Deng, Z., Li, M., et al. (2016) Phosphorus-Doped Carbon Nitride Tubes with a Layered Micro-Nanostructure for Enhanced Visible-Light Photocatalytic Hydrogen Evolution. Angewandte Chemie International Edition in English, 55, 1830-1834.
- 43. Hu, S., Ma, L., You, J., et al. (2014) A Simple and Efficient Method to Prepare a Phosphorus Modified G-C3N4 Visible Light Photocatalyst. RSC Advances, 4, 21657-21663.
- 44. Lan, D.H., Wang, H.T., Chen, L., et al. (2016) Phosphorous-Modified Bulk Graphitic Carbon Nitride, Facile Preparation and Application as an Acid-Base Bifunctional and Efficient Catalyst for CO2 Cycloaddition with Epoxides. Carbon, 100, 81-89.
- 45. Ma, T.Y., Ran, J., Dai, S., et al. (2015) Phosphorus-Doped Graphitic Carbon Nitrides Grown In Situ on Carbon-Fiber Paper, Flexible and Reversible Oxygen Electrodes. Angewandte Chemie International Edition in English, 54, 4646-4650. https://doi.org/10.1002/anie.201411125
- 46. Ran, J.R., Ma, T.Y., Gao, G.P., et al. (2015) Porous P-Doped Graphitic Carbon Nitride Nanosheets for Synergistically Enhanced Visible-Light Photocatalytic H2 Production. Energy & Environmental Science, 8, 3708-3717.
- 47. Zhang, L.G., Chen, X.F., Guan, J., et al. (2013) Facile Synthesis of Phosphorus Doped Graphitic Carbon Nitride Polymers with Enhanced Visible-Light Photocatalytic Activity. Materials Research Bulletin, 48, 3485-3491. https://doi.org/10.1016/j.materresbull.2013.05.040
- 48. Zhang, Y. and Antonietti, M. (2010) Photocurrent Generation by Polymeric Carbon Nitride Solids, an Initial Step towards a Novel Photovoltaic System. Asian Journal of Chemistry, 5, 1307-1311.
- 49. Zhou, Y., Zhang, L., Liu, J., et al. (2015) Brand New P-Doped G-C3N4, Enhanced Photocatalytic Activity for H2 Evolution and Rhodamine B Degradation under Visible Light. Journal of Materials Chemistry, 3, 3862-3867. https://doi.org/10.1039/C4TA05292G
- 50. Lin, Z.Z. and Wang, X.C. (2013) Nanostructure Engineering and Doping of Conjugated Carbon Nitride Semiconductors for Hydrogen Photosynthesis. Angewandte Chemie International Edition, 52, 1735-1738.
- 51. Sagara, N., Kamimura, S., Tsubota, T., et al. (2016) Photoelectrochemical CO2 Reduction by a P-Type Bo-ron-Doped G-C3n4 Electrode under Visible Light. Applied Catalysis B: Environmental, 192, 193-198. https://doi.org/10.1016/j.apcatb.2016.03.055
- 52. Wang, Y., Li, H., Yao, J., et al. (2011) Synthesis of Boron Doped Polymeric Carbon Nitride Solids and Their Use as Metal-Free Catalysts for Aliphatic C-H Bond Oxidation. Chemical Science, 2, 446-450. https://doi.org/10.1039/C0SC00475H
- 53. Yan, S.C., Li, Z.S. and Zou, Z.G. (2010) Photodegradation of Rhodamine B and Methyl Orange over Boron-Doped G-C3N4 under Visible Light Irradiation. Langmuir, 26, 3894-3901.
- 54. Lin, H., Deng, W., Zhou, T., et al. (2015) Iodine-Modified Nanocrystalline Titania for Photo-Catalytic Antibacterial Application under Visible Light Illumination. Applied Catalysis B: Environmental, 176, 36-43.
- 55. Dong, G., Zhao, K. and Zhang, L. (2012) Carbon Self-Doping Induced High Electronic Conductivity and Photoreactivity of G-C3N4. Chemical Communications, 48, 6178-6180. https://doi.org/10.1039/c2cc32181e
- 56. Fang, S., Xia, Y., Lv, K., et al. (2016) Effect of Carbon-Dots Modification on the Structure and Photocatalytic Activity of G-C3N4. Applied Catalysis B: Environmental, 185, 225-232.
- 57. Fan, Y., Tan, X., Ou, X., et al. (2016) A Novel “On-Off” Electrochemiluminescence Sensor for the Detection of Concanavalin A Based on Ag-Doped G-C3N4. Electrochimica Acta, 202, 90-99. https://doi.org/10.1016/j.electacta.2016.04.013
- 58. Deng, L. and Zhu, M. (2016) Metal Nitrogen (Co-G-C3N4) Doping of Surface-Modified Single-Walled Carbon Nanohorns for Use as an Oxygen Reduction Electrocatalyst. RSC Advances, 6, 25670-25677. https://doi.org/10.1039/C5RA27895C
- 59. Tian, J., Liu, Q., Asiri, A.M., et al. (2013) Ultrathin Graphitic Carbon Nitride Nanosheets, a Novel Peroxidase Mimetic, Fe Doping-Mediated Catalytic Performance Enhancement and Application to Rapid, Highly Sensitive Optical Detection of Glucose. Nanoscale, 5, 11604-11609.
- 60. Tonda, S., Kumar, S., Kandula, S., et al. (2014) Fe-Doped and -Mediated Graphitic Carbon Nitride Nanosheets for Enhanced Photocatalytic Performance under Natural Sunlight. Journal of Materials Chemistry A, 2, 6772. https://doi.org/10.1039/c3ta15358d
- 61. Bhunia, M.K., Yamauchi, K. and Takanabe, K. (2014) Harvesting Solar Light with Crystalline Carbon Nitrides for Efficient Photocatalytic Hydrogen Evolution. Angewandte Chemie International Edition in English, 53,11001-11005. https://doi.org/10.1002/anie.201405161
- 62. Ho, W., Zhang, Z., Lin, W., et al. (2015) Copolymerization with 2,4,6-Triaminopyrimidine for the Rolling-Up the Layer Structure, Tunable Electronic Properties, and Photocatalysis of G-C3N4. ACS Applied Materials & Interfaces, 7, 5497-5505.
- 63. Zhang, J., Zhang, M., Lin, S., et al. (2014) Molecular Doping of Carbon Nitride Photocatalysts with Tunable Bandgap and Enhanced Activity. Journal of Catalysis, 310, 24-30. https://doi.org/10.1016/j.jcat.2013.01.008
- 64. Lu, X., Xu, K., Chen, P., et al. (2014) Facile One Step Method Realizing Scalable Production of G-C3N4 Nanosheets and Study of Their Photocatalytic H2 Evolution Activity. Journal of Materials Chemistry A, 2, 18924-18928.
- 65. She, X., Liu, L., Ji, H., et al. (2016) Template-Free Synthesis of 2D Porous Ultrathin Nonmetal-Doped G-C3n4 Nanosheets with Highly Efficient Photocatalytic H2 Evolution from Water under Visible Light. Applied Catalysis B: Environmental, 187, 144-153.
- 66. Zhao, H., Yu, H., Quan, X., et al. (2014) Fabrication of Atomic Single Layer Graphitic-C3n4 and Its High Performance of Photocatalytic Disinfection under Visible Light Irradiation. Applied Catalysis B: Environmental, 152, 46-50. https://doi.org/10.1016/j.apcatb.2014.01.023
- 67. Cui, Y., Tang, Y. and Wang, X. (2015) Template-Free Synthesis of Graphitic Carbon Nitride Hollow Spheres for Photocatalytic Degradation of Organic Pollutants. Materials Letters, 161, 197-200.
- 68. He, F., Chen, G., Zhou, Y., et al. (2015) The Facile Synthesis of Mesoporous G-C3n4 with Highly Enhanced Photocatalytic H2 Evolution Performance. Chemical Communications, 51, 16244-16246.
- 69. Li, J., Cao, C. and Zhu, H. (2007) Synthesis and Characterization of Graphite-Like Carbon Nitride Nanobelts and Nanotubes. Nanotechnology, 18, Article ID: 115605.
- 70. Ma, T.Y, Dai, S., Jaroniec, M., et al. (2014) Graphitic Carbon Nitride Nanosheet-Carbon Nanotube Three-Dimensional Porous Composites as High-Performance Oxygen Evolution Electrocatalysts. Angewandte Chemie International Edition in English, 53, 7281-7285. https://doi.org/10.1002/anie.201403946
- 71. Tahir, M., Cao, C., Mahmood, N., et al. (2014) Multifunctional G-C3N4 Nanofibers, a Template-Free Fabrication and Enhanced Optical, Electrochemical, and Photocatalyst Properties. ACS Applied Materials & Interfaces, 6, 1258-1256.
- 72. Wang, J., Zhang, C., Shen, Y., et al. (2015) Environment-Friendly Preparation of Porous Graphite-Phase Polymeric Carbon Nitride Using Calcium Carbonate as Templates, and Enhanced Photoelectrochemical Activity. Journal of Materials Chemistry A, 3, 5126-5131. https://doi.org/10.1039/C4TA06778A
- 73. Wu, X., Liu, C., Li, X., et al. (2015) Effect of Morphology on the Photocatalytic Activity of G-C3N4 Photocatalysts under Visible-Light Irradiation. Materials Science in Semiconductor Processing, 32, 76-81. https://doi.org/10.1016/j.mssp.2014.11.047
- 74. Yang, J., Wu, X., Li, X., et al. (2011) Synthesis and Characterization of Nitrogen-Rich Carbon Nitride Nanobelts by Pyrolysis of Melamine. Applied Physics A, 105, 161-166.
- 75. Sun, Z., Li, C., Yao, G., et al. (2016) In Situ Generated G-C3n4/Tio2 Hybrid over Diatomite Supports for Enhanced Photodegradation of Dye Pollutants. Material Design, 94, 403-409.
- 76. Wang, W., Yu, J.C., Xia, D., et al. (2013) Graphene and G-C3n4 Nanosheets Cowrapped Elemental Alpha-Sulfur as a Novel Metal-Free Heterojunction Photocatalyst for Bacterial Inactivation under Visible-Light. Environmental Science & Technology, 47, 8724-8732. https://doi.org/10.1021/es4013504
- 77. Zhang, J., Zhang, M., Sun, R.Q., et al. (2012) A Facile Band Alignment of Polymeric Carbon Nitride Semiconductors to Construct Isotype Heterojunctions. Angewandte Chemie International Edition in English, 51, 10145-10149.
- 78. Zhang, S, Li, J., Wang, X., et al. (2014) In Situ Ion Exchange Synthesis of Strongly Coupled Ag@AgCl/g-C3N4 Porous Nanosheets as Plasmonic Photocatalyst for Highly Efficient Visible-Light Photocatalysis. ACS Applied Materials & Interfaces, 6, 22116-22125.
- 79. Zhang, Y., Wen, R., Guo, D., et al. (2016) One-Step Facile Fabrication and Photocatalytic Activities of ZnS@g-C3N4 Nanocomposites from Sulfatotris (Thiourea) Zinc(II) Complex. Applied Organometallic Chemistry, 30, 160-166.
- 80. Li, Z., Wang, J., Zhu, K., et al. (2015) Ag/G-C3N4 Composite Nanosheets, Synthesis and Enhanced Visible Photocatalytic Activities. Materials Letters, 145, 167-170. https://doi.org/10.1016/j.matlet.2015.01.058
- 81. Liu, L., Qi, Y., Yang, J., et al. (2015) An AgI@g-C3N4 Hybrid Core@Shell Structure,Stable and Enhanced Photocatalytic Degradation. Applied Surface Science, 358, 319-327.
- 82. Liu, S., Sun, H., O'donnell, K., et al. (2016) Metal-Free Melem/G-C3n4 Hybrid Photocatalysts for Water Treatment. Journal of Colloid and Interface Science, 464, 10-17. https://doi.org/10.1016/j.jcis.2015.11.003
- 83. Liu, X., Jian, X., Yang, H., et al. (2016) A Novel Method for Evaluating the Photoelectrocatalytic Performance of Reduced Graphene Oxide/Protonated G-C3N4 Composites. Materials Letters, 176,209-212.
- 84. Ma, H., Li, Y., Li, S., et al. (2015) Novel PO Codoped G-C3n4 with Large Specific Surface Area, Hydrothermal Synthesis Assisted by Dissolution-Precipitation Process and Their Visible Light Activity under Anoxic Conditions. Applied Surface Science, 357, 131-138.
- 85. Huang, Z., Li, F., Chen, B., et al. (2015) Porous and Low-Defected Graphitic Carbon Nitride Nanotubes for Efficient Hydrogen Evolution under Visible Light Irradiation. RSC Advances, 5, 102700-102706.
- 86. Hu, S., Ma, L., Xie, Y., et al. (2015) Hydrothermal Synthesis of Oxygen Functionalized S-P Codoped G-C3N4 Nanorods with Outstanding Visible Light Activity under Anoxic Conditions. Dalton Transactions, 44, 20889-20897. https://doi.org/10.1039/C5DT04035C
- 87. Ye, S., Wang, R., Wu, M.Z., et al. (2015) A Review on G-C3N4 for Photocatalytic Water Splitting and CO2 Reduction. Applied Surface Science, 358, 15-27.
- 88. Jun, Y.S., Park, J., Lee, S.U., et al. (2013) Three-Dimensional Macroscopic Assemblies of Low-Dimensional Carbon Nitrides for Enhanced Hydrogen Evolution. Angewandte Chemie International Edition in English, 52, 11083-11087.
- 89. Jun, Y.S., Lee, E.Z., Wang, X., et al. (2013) From Melamine-Cyanuric Acid Supramolecular Aggregates to Carbon Nitride Hollow Spheres. Advanced Functional Materials, 23, 3661-3667.
- 90. Shen, J.S., Cai, Q.G., Jiang, Y.B., et al. (2010) Anion-Triggered Melamine Based Self-Assembly and hydrogel. Chemical Communications, 46, 6786-6788. https://doi.org/10.1039/c0cc02030c
- 91. You, R., Dou, H.L., Chen, L., Zheng, S.H. and Zhang, Y.P. (2017) Graphitic Carbon Nitride with S and O Codoping for Enhanced Visible Light Photocatalytic Performance. RSC Advances, 7, 15842-15850.