Journal of Advances in Physical Chemistry
Vol.04 No.02(2015), Article ID:15361,10
pages
10.12677/JAPC.2015.42009
Self-Assembled Growth of Ordered CuPc Nanostructures
Li Song, Xuan Wang, Lu Chen, Huanhuan Song, Yongping Zhang*
Faculty of Materials and Energy, Southwest University, Chongqing
*通讯作者。
Email: *zhangyyping@yahoo.com
Received: May 8th, 2015; accepted: May 23rd, 2015; published: May 28th, 2015
Copyright © 2015 by authors and Hans Publishers Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



ABSTRACT
Copper Phthalocyanine (CuPc) has been found extensive application in gas sensors, photoelectric transistors and solar cells due to its unique molecular structure and excellent photoelectrical properties. The molecular alignment and orientation in the organic layers affect the performance of devices to a great extent. This paper reviews the self-assembly growth of CuPc layers on metal and graphite surfaces and its molecular structures by scanning tunneling microscopy (STM). The ordered CuPc monolayer is readily obtained and well characterized in ultra-high vacuum; while the self-assembled growth of ordered molecular layer in ambient environment remains a challenge. Finally, we highlight the ways to achieve stable CuPc layers at ambient environment.
Keywords:CuPc, STM, Self-Assembly, Molecular Devices

酞菁铜(CuPc)分子有序纳米结构的自组织生长
宋 礼,王 轩,陈 露,宋欢欢,张永平*
西南大学材料与能源学部,重庆
Email: *zhangyyping@yahoo.com
收稿日期:2015年5月8日;录用日期:2015年5月23日;发布日期:2015年5月28日

摘 要
酞菁铜(CuPc)因其特殊的分子结构和独特的光电性质而广泛地应用于气体传感器、光电晶体管和太阳能电池等方面。有机薄膜中分子的排列和取向会显著影响分子器件的使用性能。本文评述CuPc分子及其衍生物在金属和石墨表面的自组织生长,和使用扫描隧道显微镜(STM)研究其分子结构和性质。CuPc在超高真空环境下容易形成有序单分子层,而在大气环境下生长分子膜较为困难。但是,通过增加烷烃链,或者与稳定性好的分子混合都能在大气下得到稳定的自组织形貌。
关键词 :酞菁铜,扫描隧道显微镜,自组织生长,分子器件

1. 引言
酞菁铜(CuPc)属于芳香族分子,是由酞菁(H2Pc)分子和铜(Cu)离子形成酞菁配合物,结构如图1所示,其分子由Cu、C、N、H原子组成,中心为二价铜原子,分子4h对称,有195个价电子。CuPc为电子活性分子,结构与叶绿素相似[1] 。CuPc的稳定性、光敏性和光电导性等 [2] [3] 性质被广泛应用于气体传感器 [4] 、光电晶体管 [5] 、有机太阳能电池 [6] [7] 等薄膜器件;此外,CuPc还可以用于信息的存贮以及电极催化剂 [8] 。同时,在分子器件中,CuPc由于具有独特的电子结构和特殊的光电性质而占有一席之地 [9] [10] 。
2. 分子排列对器件性能的影响
提高酞菁的成膜技术,以得到有序的薄膜形态和结构。这将为CuPc在气体传感器中的应用开拓新的前景 [11] ,CuPc光电晶体管有机层表面的有序排列能提高其性能。分子层的垂直结构能够降低器件的驱动电压,从而优化性能,所以CuPc分子膜的有序程度显得极其重要。具有与CuPc结构相似的PbPc在太阳能电池方面的研究表明薄膜中分子结晶度和取向对太阳能电池外部量子效率的影响 [12] (如图2所示)。文中指出,在薄膜层数高于20的时候,分子与分子间的作用大于分子与基底间的作用,在相同层的PbPc薄膜的研究中表明,与基底夹角较小的薄膜层的光吸收率要高于与基底夹角大的薄膜层,这说明自组装薄膜取向对于太阳能电池的影响是非常大的。所以薄膜厚度、薄膜中分子的排列和取向对分子器件的性能有重要影响,利用自组织技术生长单层分子和多层膜是提高有机半导体器件性能的关键 [13] [14] 。
3. 扫描隧道显微镜的原理及应用
1982年G. Binnig和H. Rohrer共同研制了世界第一台新型的表面分析仪器——扫描隧道显微镜(Scanning Tunneling Microscopy,简称STM) [15] [16] 。STM的发明使得对表面研究有了新突破,研究者可以通过自己的意愿完成对单个原子的操控。对表面物理、表面化学、生命科学、材料科学和微电子技术的研究有着重大的意义和广阔的应用前景,被科学界公认为分析技术的又一次飞跃 [17] - [19] 。
STM根据探针和样品之间产生的隧道电流进行成像 [20] (图3)。针尖顶部只有一个原子,在针尖与样品间加上一个偏压,由于隧道效应,针尖与样品会产生隧道电流。这个电流与针尖同样品的距离成指数关系,电脑将这个信号吸收并形成图形。根据这个原理,扫描便有两种模式:
1) 恒高模式:保持针尖与样品的高度不变,通过记录隧道电流的值来反应形貌的高低;
2) 恒流模式:保持针尖与样品间隧道电流不变,通过改变Z轴的高度进行扫描。记录Z轴高度起伏
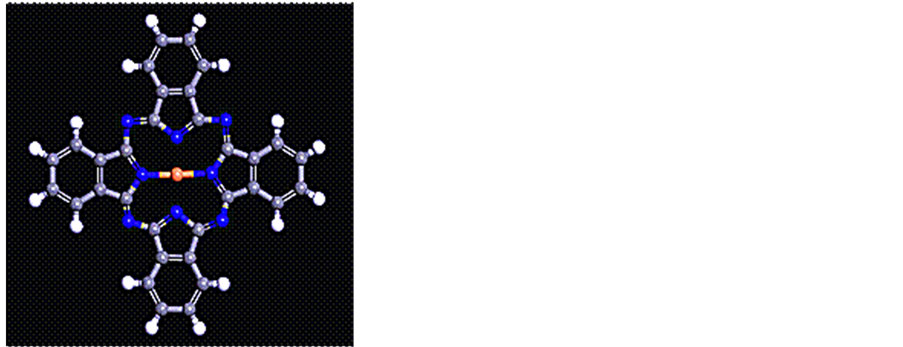
Figure 1. The molecular structure of CuPc
图1. 酞菁铜分子结构示意图
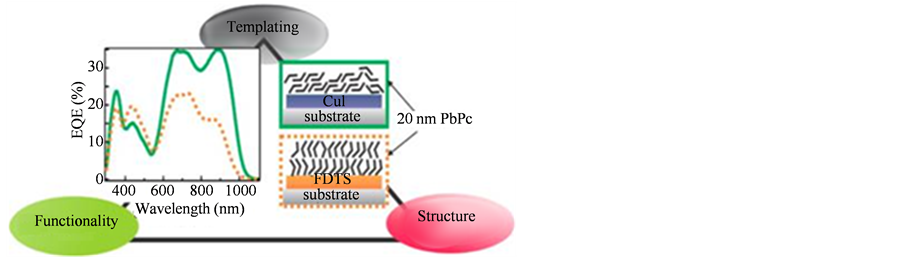
Figure 2. The external quantum efficiency of solar cells affected by the Molecular alignment and crystalline in PbPc thin film
图2. PbPc薄膜中分子结晶度和取向对太阳能电池外部量子效率的影响

Figure 3. Schematic illustration of the STM principles
图3. STM原理示意图
变化值在电脑中形成图像。
STM拥有极高的分辨率(Z向0.01 nm, X/Y向0.1 nm),可得到表面原子层的局域结构图像。对样品几乎为零损伤,对样品制备技术没有特殊的要求,除此之外,STM具有表面修饰功能,利用针尖原子对表面原子的作用力能做到对表面原子(分子)进行单个的操控 [21] 。STM可以适用多种环境:大气环境,真空环境,低温环境 [22] ,固液界面 [23] ,溶液环境,电–磁场环境等等。
4. 酞菁铜分子真空环境的自组织
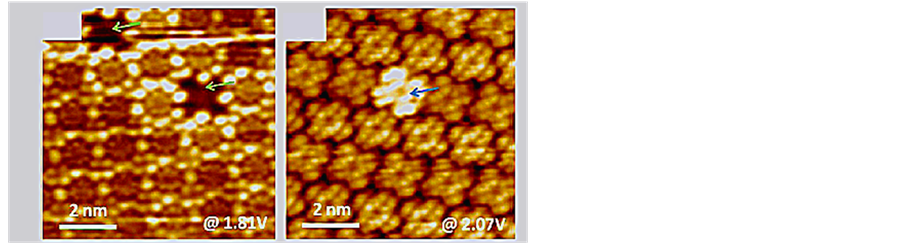
20世纪末提出的分子自组织技术,是研究纳米粒子的一种新方式,分子通过非共价键的作用(氢键、毛细力、表面张力、π-π堆积作用、阳离子吸附、疏水作用力、范德华力) [24] 自发的结合成为结构确定、热力学稳定、性能特殊的聚集体的一个过程就是分子自组织的过程。在真空利用热蒸发可以得到有序CuPc的自组织形貌 [25] 。在真空环境下扫描出的CuPc分子像 [26] (图4),通过图4,可以看出CuPc的形貌比F16CuPc的要亮很多,说明F16CuPc与石墨的结合比CuPc与石墨要稳定。
CuPc分子在HOPG上的自组织结构是平铺而非站立,通过能量越低越稳定的原理很容易解释,酞菁分子平铺时分子与基底的接触就越大,分子间通过范德华力相互吸引,使得结构非常的稳定。每一列都是整齐排列,分子间排列的也很紧密,每一列上分子非常紧密,因为CuPc分子与CuPc分子之间的范德华吸引力比静电吸引力要大,所以CuPc分子所受的横向阻力要小,分子排列紧密。CuPc分子在HOPG表面的单层生长优于多层生长,也就是说在HOPG表面CuPc分子总是倾向于一层分子的排列。原因是,在CuPc分子之间的相互作用比石墨引力弱很多,所以在石墨表面容易形成单层分子。CuPc分子中心为铜原子,每个CuPc分子都是很亮的,但是在每个亮点中心都有一个较为暗的小孔,小孔为铜原子所在的位置 [27] - [37] 。
Shi Chen等人 [38] 报道在SiC基底上的CuPc形貌图(图5),CuPc分子的尺寸为1.4 × 1.4 nm2,对角线长度为1.68 nm。但是在SiC基底上临近两个CuPc分子Cu原子的距离为1.95 nm,可以得知在SiC基底上CuPc分子相互之间的作用力可以忽略,在SiC基底上自组织的CuPc有序程度和分子间紧密程度都比在石墨上的差一些。选择基底材料对于自组织成膜也是有较大影响的,良好的基底材料可以使自组织体呈有序结构,或者是根据材料性能的要求在某个方向呈优势取向。
在高定向热解石墨上生长PTCDA分子,再在PTCDA分子上生长CuPc有序结构层。Wei Chen等人 [39] [40] 报道在PTCDA (图6)上用低温扫描隧道显微镜扫描出清晰的CuPc高度有序结构形貌,CuPc分子与分子间紧密排列存在弱的相互作用力,图6(b)中能清晰的分辨出CuPc分子平铺于PTCDA分子层上,由于中心铜原子吸附能力强而表现为小孔形状。图6(c)可以看到CuPc之间有周期性的由于CuPc覆盖层与PTCDA底层形成的位错造成的,这种现象充分说明了基底的条件对自组织具有相当大的影响,CuPc之间相互的作用力比CuPc与PTCDA层间作用力弱,也是形成这种结构的因素之一。这种具有强烈取向的自组织结构,能够形成pn有机异质结构,这在有机电子工业上有很好的应用价值。
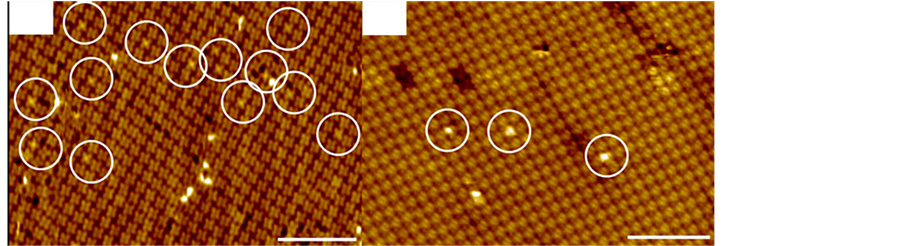
J.H. Park [41] 报道了CuPc分子的铜分子吸附NO的STM形貌(图7)。先在低温高真空中组织一层CuPc薄膜,再吸附NO。图中标尺为10 nm,白圈表示NO吸附在了CuPc上,图7(a)与图7(b)相比较,在Au表面的形貌要清晰,而且同一层中空缺的CuPc分子少,同时间(10 min)在Au表面吸附的NO也要多于HOPG。但是,同一标尺下分子个数的比较,在HOPG上组织的CuPc要密集一些,显示出金表面与CuPc分子间作用力比HOPG表面与CuPc分子间作用力强。可以通过器件的需要,在自组织的时间上进行控制,达到性能需要的结构。
5. 酞菁铜在大气环境下自组织
CuPc这类小分子在大气环境下难以扫描出清晰的以石墨为基底的STM形貌,小分子在石墨基底上通过弱的物理吸附作用形成有序结构,在大气环境下影响因素太多,加之STM在扫描过程中针尖分子与CuPc分子间形成的电流会改变原有的结构,同时STM扫描所产生的机械扰动也会有干扰。目前,还没有人报道过在大气环境下清晰的CuPc自组织形貌。
长链烷烃分子能与石墨基底形成氢键,从而增强分子的吸附能力。CuPc分子与烷烃链结合后,能在大气环境下扫面出清晰的STM形貌,烷烃链越长,分子在石墨上就越稳定。由此,在大气环境下研究者
 (a) (b)
(a) (b)
Figure 4. STM images of self-assembled F16CuPc and CuPc. (a) Two F16CuPc in CuPc, (b) a CuPc in F16CuPc
图4. F16CuPc和CuPc自组织结构的STM像。(a) CuPc中有两个F16CuPc,(b) F16CuPc中有一个CuPc
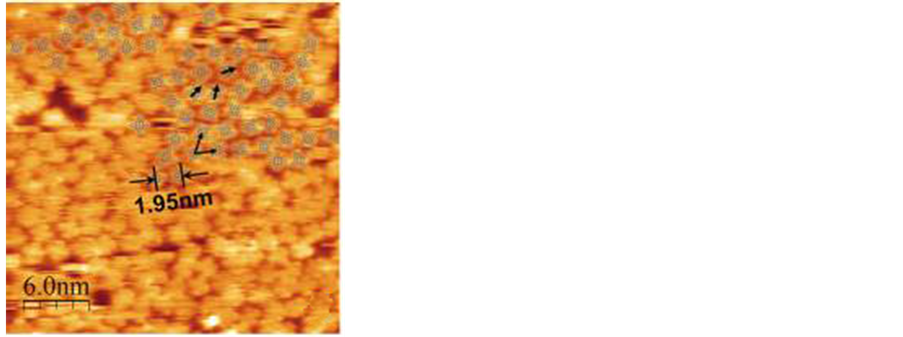
Figure 5. STM image of self-assembled CuPc on SiC
图5. 在SiC表面上CuPc的STM形貌

Figure 6. STM images of self-assembled CuPc on PTCDA
图6. 在PTCDA上自组织CuPc的STM形貌
 (a) (b)
(a) (b)
Figure 7. STM images of NO adsorption of CuPcon (a) Au surface and (b) HOPG surfaces
图7. NO吸附在CuPc的STM形貌(a) Au和(b) HOPG表面
做了大量关于基团取代CuPc分子氢的自组织研究,包括全氯取代(Cl16CuPc) [42] ,全氟取代(F16CuPc) [43] - [45] 以及各类烷基链取代的分子。这些分子由于取代基团与石墨的结合力增强,因此能在大气环境下稳定的自组织生长。
除了增加基团增强与基底结合力以外,CuPc分子与其他分子混合自组织生长也可以使其在大气环境下稳定生长。与CuPc分子混合的分子起到固定支架的作用,将CuPc分子或CuPc分子链固定在石墨基底上。
CuPcBu8和C18I分子在石墨表面交替的以链状结构排列,在石墨基底上CuPcBu8和C18I的自组织形貌如图8所示 [46] ,在标尺25 mn的尺寸里能清楚的数出分子列数,通过图8(b)放大形貌图可以清晰的看到CuPcBu8和C18I单个分子的形貌,CuPcBu8和C18I通过范德瓦尔斯力相互作用,分子比例为CuPcBu8:C18I = 1:4,分子排列相当紧密,所以此类分子能自组织稳定的结构。实验表明,两种不同分子可以同时在石墨表面形成有序的交替链式结构,而这种结构可以给材料带来新的性能。
在此之前,S.B. Lei [47] 还报道过在大气环境下CuPc和C18SH的自组织形貌如图9所示,亮区为CuPc分子链,暗区为C18SH分子链。组成分子比为CuPc比C18SH为1:3,CuPc分子间为C18SH分子,长链C18SH分子垂直CuPc分子排列,这种支架型的结构非常稳定。实验证明,CuPc分子与链烃分子的自组织结构能够稳定的抵抗大气中各种影响因素和STM扫描带来的机械干扰。CuPc分子以长链形式排列,具有高度选择性,这种特殊的性质在半导体材料中有很大的应用价值。
殷淑霞 [48] 等人报道了CuPcOC8在大气环境下的高分辨STM形貌,如图10所示。图的尺寸为14 × 14 nm2,图中能明显的看出酞菁部分,即亮的区域;烷基部分则相对较暗。烷基势能较低,能与石墨较为紧密的吸附,使得CuPcOC8分子也较为稳定,所以在大气环境下能扫描出高分辨的高度有序的形貌。如果小分子加上烷基链还是表现出该分子的性质,那么在自组织就可以被替代,在大气环境下能够自组织成有序结构更易于工业化生产。同时结构更加稳定带来的是器件寿命的延长,所以这个方向也是一个有前途的研究方向。
前面报道真空下CuPc在Ag表面自组织结构,是规则的链式排列组合。而最近根据G. Antczak [49] 报道,在大气环境下CuPc在Ag表面自组织结构与真空下有所不同,如图11所示,根据图11(a)可以,可以清晰的看见CuPc分子之间有小点,而该小点就是金属Ag。G. Antczak解释Ag可以轻松的吸附在CuPc分子排列后留下的空隙里,这种吸附行为能增加CuPc分子间的联系,把单个CuPc分子联系在一起形成如图11(b)所示的(CuPc)4Ag和(CuPc)6Ag2结构。Ag的吸附,增加了CuPc结合力和稳定性。通过对表面的轰击处理,改变表面状态,自组装生长结构也会改变。
基底表面的原子结构对CuPc的生长有着巨大的影响。比如CuPc/ATO晶体管的性能要优于CuPc/SiO2

Figure 8. STM images of CuPcBu8/C18I on HOPG. (a) Large scanning area and (b) high-resolution
图8. CuPcBu8/C18I的STM形貌,(a) 大扫描范围和(b) 高分辨
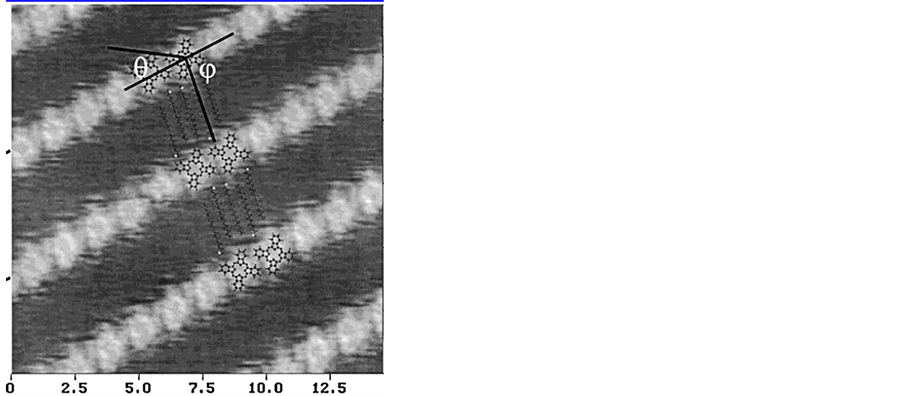
Figure 9. STM image of CuPc/C18SH on HOPG
图9. CuPc/C18SH的STM形貌
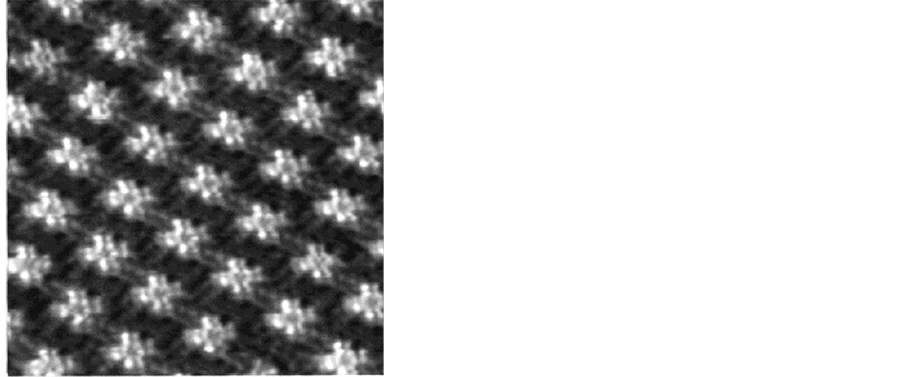
Figure 10. STM image of CuPcOC8 on HOPG
图10. CuPcOC8的高分辨STM形貌

Figure 11. (a) STM image of CuPc on Ag, and (b) STM image of (CuPc)4Ag and (CuPc)6Ag2
图11. (a) CuPc在Ag的STM形貌和(b) (CuPc)4Ag和(CuPc)6Ag2的STM形貌
[50] 。因为在相同薄膜层数的CuPc/ATO晶体管的掺杂浓度要低于CuPc/SiO2,同时CuPc在ATO表面测得的流动性也高于SiO2;在MoS2表面上,CuPc分子平行于基底表面形成均匀连续的单分子层和薄膜 [51] 。薄膜的厚度和沉积的温度对薄膜的形貌影响较大,在沉积层数较少的情况下,温度在330 K以下,CuPc分子以站立方式沉积,温度在450 K以上,CuPc分子以平铺方式沉积。
6. 结论与展望
CuPc具有独特的分子结构以及特殊的光电性质,在有机太阳能电池、光电晶体管、气体传感器及分子器件等方面得以广泛应用。分子的排列方式以及有序化程度对器件的性质有很大的影响,CuPc在超高真空环境下容易形成有序单分子层,而在大气环境下生长分子膜较为困难。
在大气环境下自组织结构不稳定,需要通过取代增加烷烃或者与其他分子混合组织增加其稳定性。另外,选择合适的基体表面和表面修饰对形成稳定有序CuPc薄膜有重要影响。如果能在大气环境下能得到稳定的CuPc自组织薄膜,将会使得CuPc太阳能电池、光电晶体管等的生产工艺简单和器件造价降低,对于纳米材料和能源应用有重大的影响。
基金项目
中央高校基本科研业务费专项资金资助,编号:XDJK2015D001。
文章引用
宋 礼,王 轩,陈 露,宋欢欢,张永平, (2015) 酞菁铜(CuPc)分子有序纳米结构的自组织生长
Self-Assembled Growth of Ordered CuPc Nanostructures. 物理化学进展,02,66-76. doi: 10.12677/JAPC.2015.42009
参考文献 (References)
- 1. Lu, X., Hipps, K.W., Wang, X.D. and Mazur, U. (1996) Scanning tunneling microscopy of metal phthalocyanines:d(7) and d(9) Cases. Journal of the American Chemical Society, 30, 7197-7202.
- 2. Claessens, C.G., Hahn, U. and Torres, T. (2008) Phthalocyanines: from outstanding electronic properties to emerging applications. Chemical Record, 8, 75-97.
- 3. Jiang, N., Foley, E.T., Klingsporn, J.M., Sonntag, M.D., Valley, N.A., Dieringer, J.A., Seideman, T., Schatz, G.C., Hersam, M.C. and Van Duyne, R.P. (2012) Observation of multiple vibrational modes in ultrahigh vacuum tip-en- hanced raman spectroscopy combined with molecular-resolution scanning tunneling microscopy. Nano Letters, 10, 5061-5067.
- 4. Bohrer, F.I., Colesniuc, C.N., Park, J., Ruidiaz, M.E., Schuller, I.K., Kummel, A.C. and Trogler, W.C. (2009) Comparative gas sensing in cobalt, nickel, copper, zinc and metal-free phthalocyanine chemiresistors. Journal of the American Chemical Society, 131, 478-485.
- 5. Gao, D.M., Zhang, X., Kong, X., Chen, Y.L. and Jiang, J.Z. (2015) (TFPP)Eu[Pc(OPh)8]Eu[Pc(OPh)8]/CuPc two- component bilayer heterojunction-based organic transistors with high ambipolar performance. ACS Applied Materials and Interfaces, 4, 2486-2493.
- 6. Priya, J.J., Aseema M., Jason S., Lee, J.Y. and Baldo, M.A. (2011) Singlet exciton fission in nanostructured organic solar cells. Nano Letters, 4, 1495-1498.
- 7. Dutton, G.J. and Robey, S.W. (2012) Exciton dynamics at CuPc/C60 interfaces: Energy dependence of exciton dissociation. Journal of Physical Chemistry C, 36, 19173-19181.
- 8. Shrestha, N.K., Kohn, H., Imamura, M., Irie, K., Ogihara, H. and Saji, T. (2010) Electrophoretic deposition of phthalocyanine in organic solutions containing trifluoroacetic acid. Langmuir, 22, 17024-17027.
- 9. Berkelaar, R.P., Sode, H., Mocking, T.F., Kumar, A., Poelsema, B. and Zandvliet, H.J.W. (2011) Molecular bridges. Journal of Physical Chemistry C, 5, 2268-2272.
- 10. Heitzer, H.M., Marks, T.J. and Ratner, M.A. (2014) Maximizing the dielectric response of molecular thin films via quantum chemical design. ACS Nano, 12, 12587-12600.
- 11. Su, Y.R., Ouyang, M., Liu, P.Y., Luo, Z., Xie, W.G. and Xu, J.B. (2013) Insights into the interfacial properties of low-voltage CuPc field-effect transistor. ACS Applied Materials and Interfaces, 11, 4960-4965.
- 12. Vasseur, K., Broch, K., Ayzner, A.L., Rand, B.P., Cheyns, D., Frank, C., Schreiber, F., Toney, M.F., Froyen, L. and Heremans, P. (2013) Controlling the texture and crystallinity of evaporated lead phthalocyanine thin films for near- infrared sensitive solar cells. ACS Applied Materials and Interfaces, 17, 8505-8515.
- 13. Yamada, T., Shibuta, M., Ami, Y., Takano, Y., Nonaka, A., Miyakubo, K. and Munakata, T. (2010) Novel growth of naphthalene overlayer on Cu(111) studied by STM, LEED, and 2PPE. Journal of Physical Chemistry C, 31, 13334- 13339.
- 14. Pomerantz, M., Aviram, A., McCorkle, R.A., Li, L. and Schrott, A.G. (1992) Rectification of STM current to graphite covered with phthalocyanine molecules. Science, 2, 1115-1118.
- 15. Weiss, P.S. (2009) Nanoscience challenges. ACS Nano, 4, 753-754.
- 16. Chan, W.C.W., Gogotsi, Y., Hafner, J.H., Moehwald, H., Hammond, P.T., Mulvaney, P.A., Hersam, M.C., Nel, A.E., Javey, A., Nordlander, P.J., Kagan, C.R., Parak, W.J., Khademhosseini, A., Penner, R.M., Kotov, N.A., Rogach, A.L., Lee, S.T., Schaak, R.E., Stevens, M.M., Willson, C.G., Wee, A.T.S. and Weiss, P.S. (2014) A year for nanoscience. ACS Nano, 12, 11901-11903.
- 17. Swart, I., Sonnleitner, T., Niedenfuhr, J. and Repp, J. (2012) Controlled lateral manipulation of molecules on insulating films by STM. Nano Letters, 2, 1070-1074.
- 18. Shapir, E., Sagiv, L., Borovok, N., Molotski, T., Kotlyar, A.B. and Porath, D. (2008) High-resolution STM imaging of novel single G4-DNA molecules. Journal of Physical Chemistry B, 31, 9267-9269.
- 19. Yang, Y.C., Taranovskyy, A. and Magnussen, O.M. (2012) In situ video-STM studies of methyl thiolate surface dynamics and self-assembly on Cu(100) electrodes. Langmuir, 40, 14143-14154.
- 20. Tersoff, J. and Hamann, D. (1983) Theory and application for the scanning tunneling microscope. Physical Review Letters, 25, 1998-2001.
- 21. Hansma, P.K., Elings, V.B., Marti, O. and Bracker, C.E. (1988) Scanning tunneling microscopy and atomic force microscopy: Application to biology and technology. Science, 8, 209-216.
- 22. Zhang, Y.F., Isshiki, H., Katoh, K., Yoshida, Y., Yamashita, M., Miyasaka, H., Breedlove, B.K., Kajiwara, T., Takaishi, S. and Komeda, T. (2009) A low-temperature scanning tunneling microscope investigation of a nonplanar dysprosium phthalocyanine adsorption on Au(111). Journal of Physical Chemistry C, 32, 14407-14410.
- 23. Feyter, S.D. and Schryver, F.C.D. (2005) Self-assembly at the liquid/solid interface: STM reveals. Journal of Physical Chemistry B, 10, 4290-4302.
- 24. Whitesides, G.M., Mathias, J.P. and Seto, C.T. (1991) Molecular self-assembly and nanochemistry: A chemical strategy for the synthesis of nanostructures. Science, 254, 1312-1319.
- 25. Zang, C.Y., Jia, Z.X., Chen, Q.Y., Zhang, L., Si, Z.J., Song, J., Wang, X.W. and Lu, J.W. (2008) Self-assembled growth and photoluminescence of leaf-like Zn/ZnO microstructure by thermal e-vaporation method. Chinese Journal of Luminescence, 6, 945-949.
- 26. Zhong, J.Q., Qin, X.M., Zhang, J.L., Kera, S., Ueno, N., Wee, A.T.S., Yang, J.L. and Chen, W. (2014) Energy level realignment in weakly interacting donor acceptor binary molecular networks. ACS Nano, 2, 1699-1707.
- 27. Yin, S.X., Wang, C., Xu, B. and Bai, C.L. (2002) Studies of CuPc adsorption on graphite surface and alkane adlayer. Journal of Physical Chemistry B, 106, 9044-9047.
- 28. Wang, S.D., Dong, X., Lee, C.S. and Lee, S.T. (2004) Orderly growth of copper phthalocyanine on HOPG at high substrate temperatures. Journal of Physical Chemistry B, 108, 1529-1532.
- 29. Ogawa, Y., Niu, T.C., Wong, S.L., Tsuji, M., Wee, A.T.S., Chen, W. and Ago, H. (2013) Self-assembly of polar phthalocyanine molecules on graphene grown by chemical vapor deposition. Journal of Physical Chemistry C, 117, 21849-21855.
- 30. Otsuki, J., Kawaguchi, S., Yamakawa, T., Asakawa, M. and Miyake, K. (2006) Arrays of double-decker porphyrins on highly oriented pyrolytic. Langmuir, 22, 5708-5715.
- 31. Ikeda, T., Asakawa, M., Miyake, K., Goto, M. and Shimizu, T. (2008) Scanning tunneling microscopy observation of self-assembled monolayers of strapped porphyrins. Langmuir, 24, 12877-12882.
- 32. Zheng, Q.N., Wang, L., Zhong, Y.W., Liu, X.H., Chen, T., Yan, H.J., Wang, D., Yao, J.N. and Wan, L.J. (2014) Adaptive reorganization of 2D molecular nanoporous network induced by coadsorbed guest molecule. Langmuir, 30, 3034- 3040.
- 33. Ahlund, J., Schnadt, J., Nilson, K., Gothelid, E., Schiessling, J., Besenbacher, F., Martensson, N. and Puglia, C. (2007) The adsorption of iron phthalocyanine on graphite: A scanning tunneling microscopy study. Surface Science, 601, 3661-3667.
- 34. Antczak, G., Kaminski, W. and Morgenstern, K. (2015) Morgenstern stabilizing CuPc coordination networks on Ag(100) by Ag atoms. Journal of Physical Chemistry C, 3, 1442-1450.
- 35. Xiao, K., Deng, W., Keum, J.K., Yoon, M., Vlassiouk, I.V., Clark, K.W., Li, A.P., Kravchenko, I.I., Gu, G., Payzant, E.A., Sumpter, B.G., Smith, S.C., Browning, J.F. and Geohegan, D.B. (2013) Surface-induced orientation control of CuPc molecules for the epitaxial growth of highly ordered organic crystals on graphene. Journal of the American Chemical Society, 9, 3680-3687.
- 36. de Oteyza, D.G., Garcia-Lastra, J.M., Goiri, E., El-Sayed, A., Wakayama, Y. and Ortega, J.E. (2014) Asymmetric response toward molecular fluorination in binary copper-phthalocyanine/pentacene assemblies. Journal of Physical Chemistry C, 32, 18626-18630.
- 37. Sumpter, B.G., Liang, L.B., Nicolai, A. and Meunier, V. (2014) Interfa-cial properties and design of functional energy materials. Accounts of Chemical Research, 11, 3395-3405.
- 38. Chen, S., Chen, W., Huang, H., Gao, X.Y., Qi, D.C., Wang, Y.Z. and Wee, A.T.S. (2010) Template-directed molecular assembly on silicon carbide nanomesh: Comparison between CuPc and pentacene. ACS Nano, 2, 849-854.
- 39. Chen, W., Huang, H., Chen, S., Gao, X.Y. and Wee, A.T.S. (2008) Low-temperature scanning tunneling microscopy and near-edge X-ray absorption fine structure investigations of molecular orientation of copper(II) phthalocyanine thin films at organic heterojunction interfaces. Journal of Physical Chemistry C, 112, 5036-5042.
- 40. Huang, Y.L., Li, H., Ma, J., Huang, H., Chen, W. and Wee, A.T.S. (2010) Scanning tunneling microscopy investigation of self-assembled CuPc/F16CuPc binary superstructures on graphite. Langmuir, 5, 3329-3334.
- 41. Park, J.H., Choudhry, P. and Kummel, A.C. (2014) NO adsorption on copper phthalocyanine functionalized graphite. Journal of Physical Chemistry C, 19, 10076-10082.
- 42. Lei, S.B., Deng, K., Yang, D.L., Zeng, Q.D. and Wang, C. (2006) Charge-transfer effect at the interface of phthalocyanine-electrode contact studied by scanning tunneling spectroscopy. Journal of Physical Chemistry B, 3, 1256-1260.
- 43. Zhang, K.H.L., Li, H., Mao, H.Y., Huang, H., Ma, J., Wee, A.T.S. and Chen, W. (2010) Control of two-dimensional ordering of F16CuPc on Bi/Ag(111): Effect of interfacial interactions. Journal of Physical Chemistry C, 25, 11234- 11241.
- 44. El-Sayed, A., Mowbray, D.J., Garcia, L.J.M., Rogero, C., Goiri, E., Borghetti, P., Turak, A., Doyle, B.P., Dell, A.M., Floreano, L., Wakayama, Y., Rubio, A., Ortega, J.E. and de Oteyza, D.G. (2012) Supramolecular environment-de- pendent electronic properties of metal-organic interfaces. Journal of Physical Chemistry C, 7, 4780-4785.
- 45. Hasegawa, Y., Yamada, Y. and Sasaki, M. (2014) Reordering and disordering of the copper hexadecafluorophthalocyanine (F16CuPc) monolayer by K doping. Journal of Physical Chemistry C, 42, 24490-24496.
- 46. Lei, S.B., Yin, S.X., Wang, C., Wan, L.J. and Bai, C.L. (2002) Modular assembly of al-kyl-substituted phthalocyanines with 1-iodooctadecane. Chemistry of Materials, 14, 2837-2838.
- 47. Lei, S.B., Wang, C., Yin, S.X. and Bai, C.L. (2001) Single molecular arrays of phthalocyanine assembled with nanometer sized alkane templates. Journal of Physical Chemistry B, 105, 12272-12277.
- 48. Qiu, X.H., Wang, C., Yin, S.X., Zeng, Q.D., Xu, B. and Bai, C.L. (2000) Self-assembly and immobilization of metallophthalocyanines by alkyl substituents observed with scanning tunneling microscopy. Journal of Physical Chemistry B, 15, 3570-3574.
- 49. Antczak, G., Kaminski, W. and Morgenstern, K. (2015) Stabilizing CuPc coordination networks on Ag(100) by Ag atoms. Journal of Physical Chemistry C, 119, 1442-1450.
- 50. Su, Y.R., Ouyang, M., Liu, P.Y., Luo, Z., Xie, W.G. and Xu, J.B. (2013) Insights into the interfacial properties of low-voltage CuPc field-effect transistor. ACS Applied Materials and Interfaces, 5, 4960-4965.
- 51. Alvarez, L., Fall, F., Belhboub, A., Le Parc, R., Almadori, Y., Arenal, R., Aznar, R., Dieu-donne-Geroge, P., Hermet, P., Rahmani, A., Jousselme, B., Campidelli, S., Cambedouzou, J., Saito, T. and Bantignies, J.L. (2015) One-dimensional molecular crystal of phthalocyanine confined into single-walled carbon nanotubes. Journal of Physical Chemistry C, 119, 4217-4223.